
Why water is a compound and not a mixture? What's Insight
A compound contains atoms of different elements chemically combined together in a fixed ratio. A mixture is a combination of two or more substances where there is no chemical combination or reaction. Compounds contain different elements in a fixed ratio arranged in a defined manner through chemical bonds. They contain only one type of molecule.
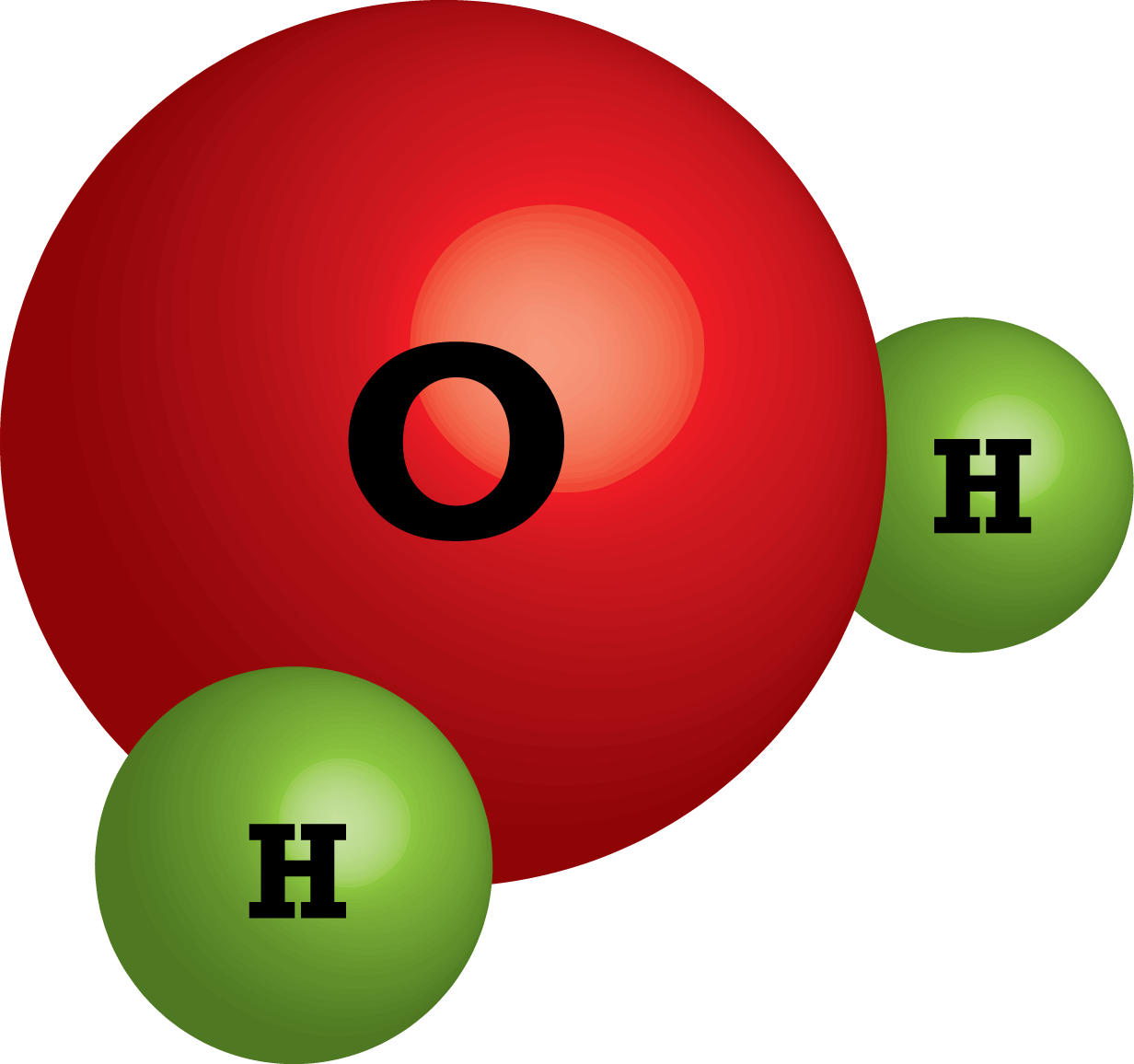
Is Water An Element Or A Compound My XXX Hot Girl
A compound is a substance that contains atoms of two or more different elements, chemically joined together. For example, water is a compound of hydrogen and oxygen, chemically joined together.
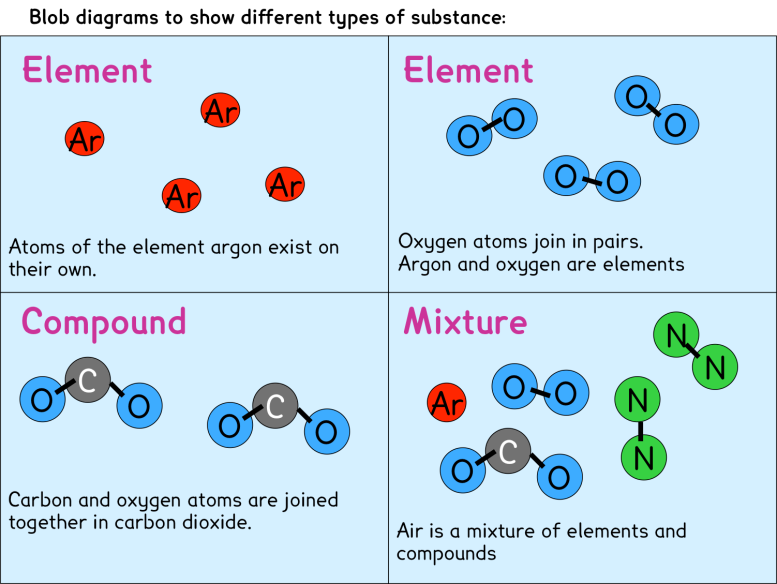
1.8 Elements, compounds, mixtures Chemistry
Yes, pure water is a compound. However, pure water rarely exists on Earth. As a result, if the water contains any 'extras,' it will be considered a mixture. And water almost always contains 'extras.'.

Is Water an Element or a Compound?
Mixtures can always be separated again into component pure substances,because bonding among the atoms of the constituent substances does not occur in a mixture. Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their individual properties. For example sodium is a soft shiny.

PPT PURE SUBTANCES AND MIXTURES PowerPoint Presentation, free download ID2120618
5. A mixture is when two or more substances combine physically together. However, in water, two hydrogen atoms combine with one oxygen atom chemically, forming a new substance that has properties different from hydrogen alone or oxygen alone. For example, if you combine iron powder and sulfur powder physically (just mixing them together without.

Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo
Classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition;. When heated in the absence of air, the compound sucrose is broken down into the element carbon and the compound water. (The initial stage of this process, when the sugar is turning brown, is known as.
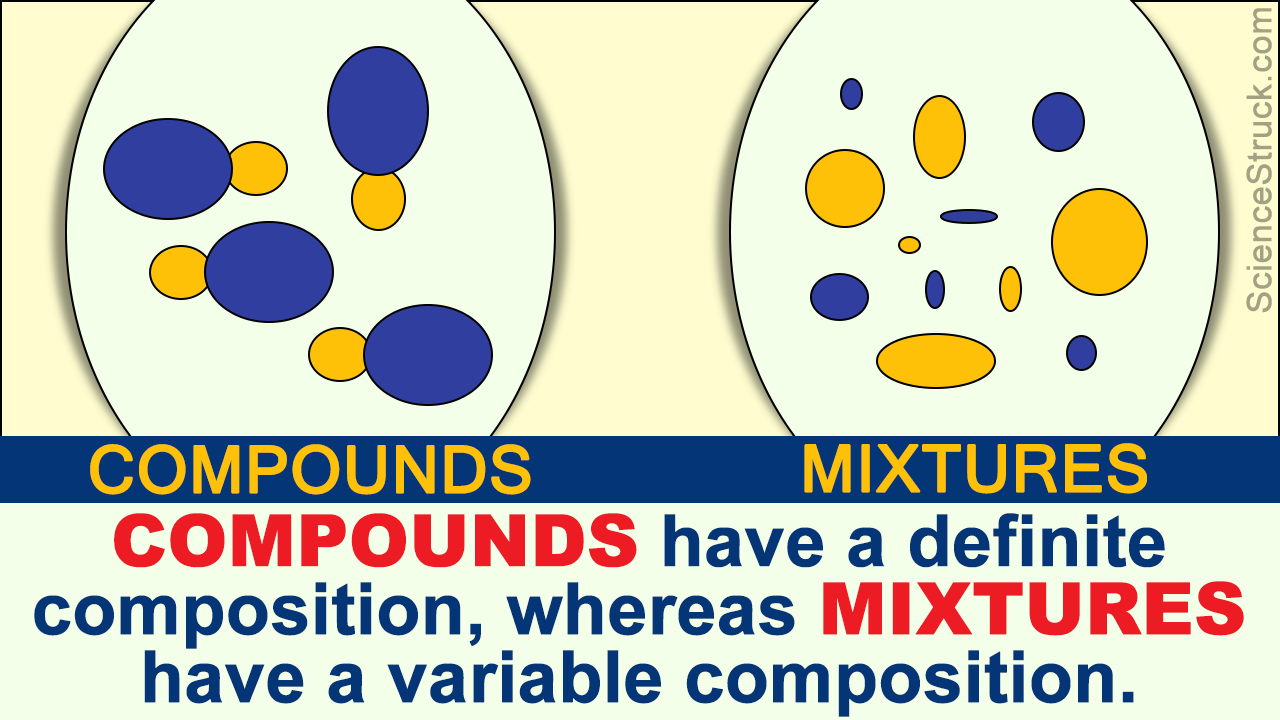
Differences Between Compounds and Mixtures
A mixture refers to a physical combination of two or more substances, where no reaction occurs. Because only a physical change occurred, a mixture can separate back into its original components. For example, saltwater is a mixture. When you boil it, the water evaporates while the salt stays behind. Others include salt and oil, cereal in milk.
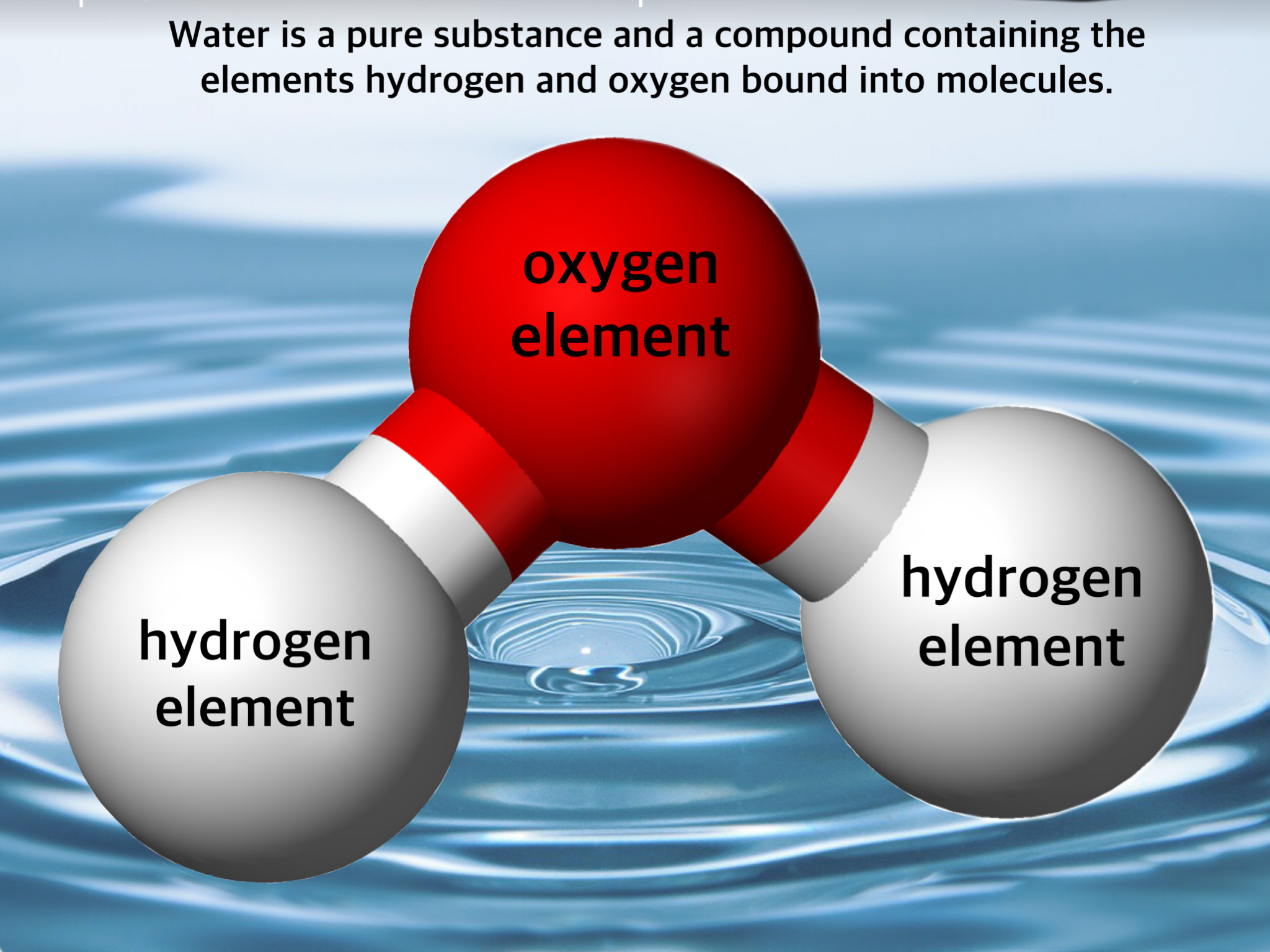
1.2 What Chemists Do Chemistry LibreTexts
A water molecule comprises two hydrogen atoms and one oxygen atom that chemically combines through a covalent bond. We know that hydrogen and oxygen atoms are gases, and when they combine to form a water molecule, it changes its state to liquid. Hence, water is a compound, or a pure substance, with entirely different properties than its elements.
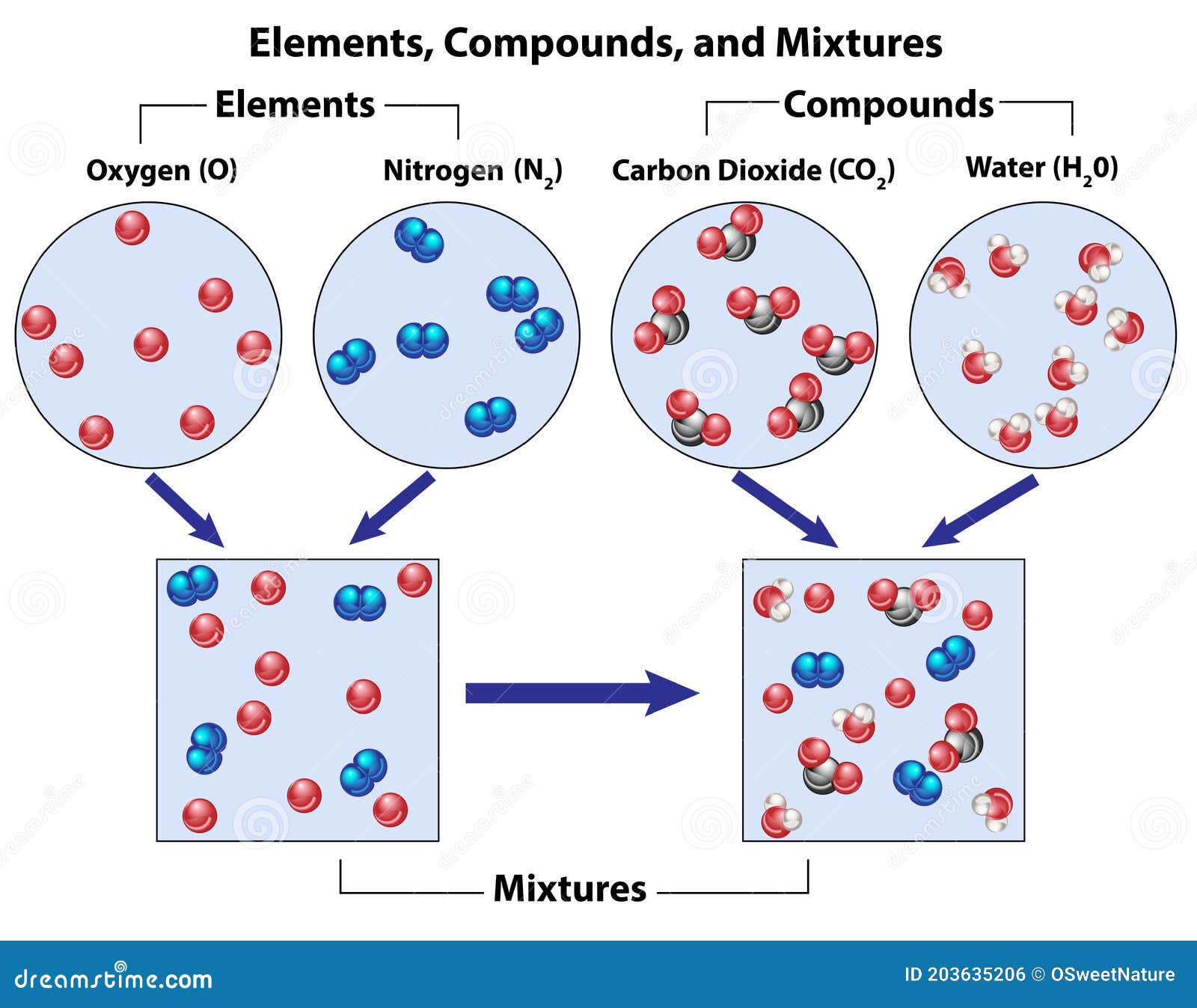
Element Compound And Mixture Chart
A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. Often it is easy to confuse a homogeneous mixture with a pure substance, because they are both uniform.
The amount of water on Earth is a constant
Mixtures. A mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by physical changes, such as evaporation (you will learn more about this later). A mixture with a composition that varies from point to point is called a heterogeneous mixture.Italian dressing is an example of a heterogeneous mixture (Figure 4).

PPT Chapter 1 Elements , Compounds, and Mixtures PowerPoint Presentation ID1852305
Water as a Compound and Molecule . A compound forms whenever two or more atoms form chemical bonds with each other. The chemical formula for water is H 2 O, which means each molecule of water consists of one oxygen atom chemically bonded to two hydrogen atoms. Thus, water is a compound. It's also a molecule, which is any chemical species formed by two or more atoms chemically bonded to each other.

is water a element compound or mixture YouTube
Identify the following combinations as heterogeneous mixtures or homogenous mixtures. soda water (Carbon dioxide is dissolved in water.) a mixture of iron metal filings and sulfur powder (Both iron and sulfur are elements.) Figure \(\PageIndex{4}\): A mixture of iron filings and sulfur powder (Asoult, Fe-S mixture 03, CC BY 4.0) Solution

Mixtures vs. Compounds What is a compound ChemTalk
water, a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid states. It is one of the most plentiful and essential of compounds.A tasteless and odourless liquid at room temperature, it has the important ability to dissolve many other substances. Indeed, the versatility of water as a solvent is essential to living organisms.

images of elements compounds and mixtures differences Brainly.in
Homogeneous Mixtures. A homogeneous mixture has a uniform composition, meaning that if several samples are taken, each would contain the same chemicals in approximately the same ratio.Salt water is an example of a homogeneous mixture, as it contains two distinctive chemicals, a "salt," which is most likely sodium chloride, NaCl, and water, H 2 O. These compounds can be separated by boiling the.
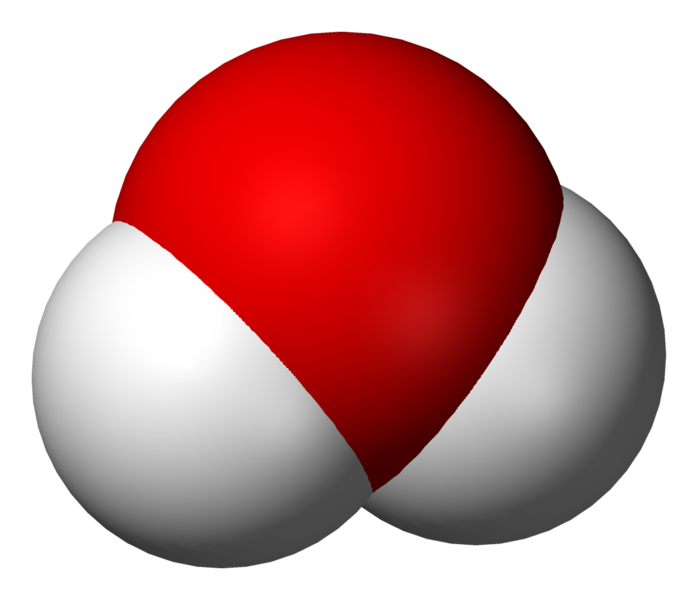
is h2o a compound
Water is both a molecule and a compound. Why Water Is not a Mixture. Another common question is whether water is a mixture. The thinking is that since water consists of a combination of hydrogen and oxygen atoms, it must be a mixture. However, scientists define a mixture to be a material produced when two substances are physically combined.
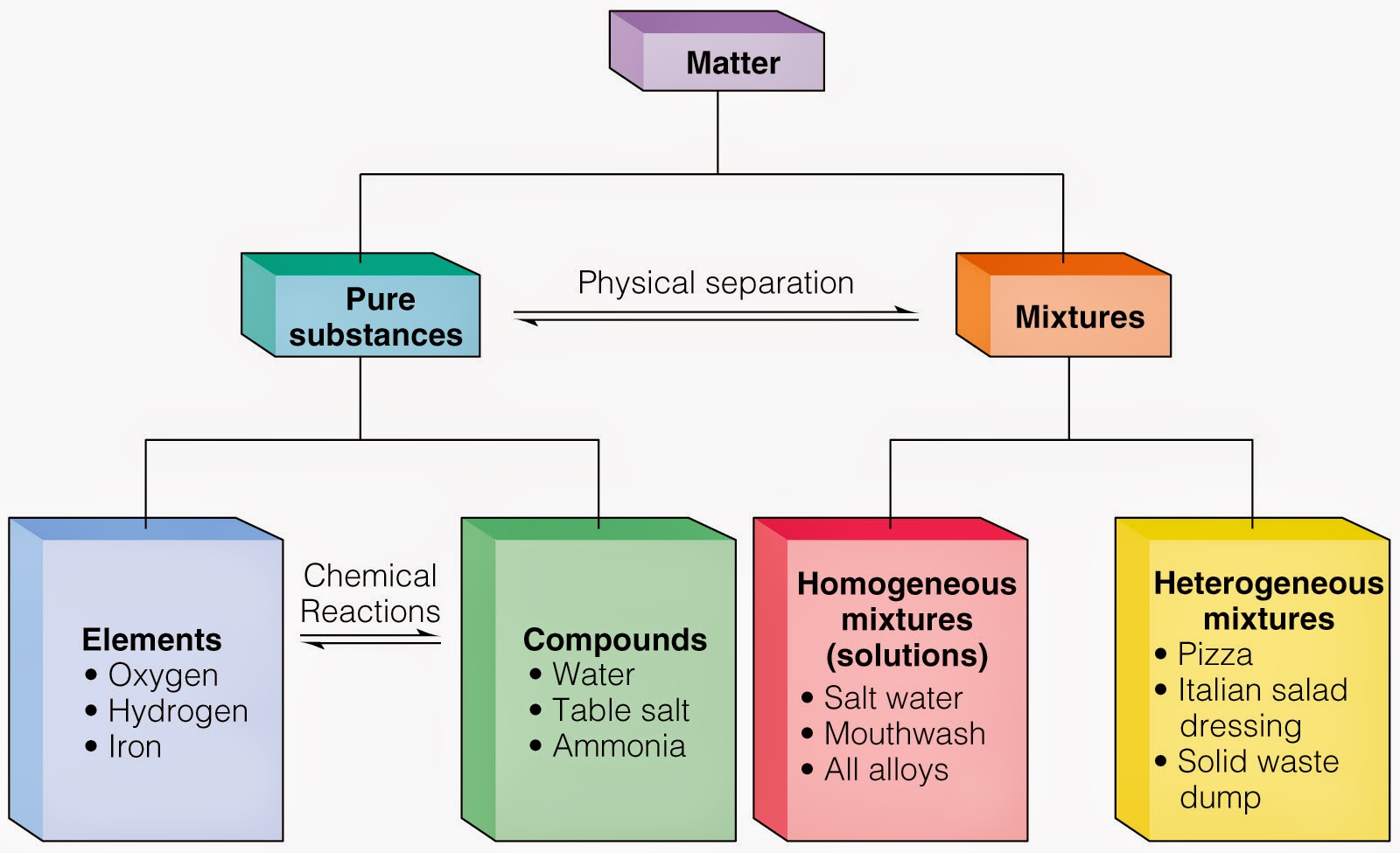
6th Grade Science 5th Six Weeks (Wk 1 & 2) Matter Pure Substances and Mixtures Elements and
A heterogeneous mixture is a mixture of two or more chemical substances (elements or compounds) where the different components can be visually distinguished and easily separated by physical means. Examples include: mixtures of sand and water; mixtures of sand and iron filings; a conglomerate rock; water and oil; a salad; trail mix
- Lay All Your Love On Me Abba
- Tom Tate Gold Coast Mayor
- Construction Material Store Near Me
- New Zealand Mobile Number Format
- Old Fire Station Backpackers Fremantle
- Second World War Movies Netflix
- Youtube Chaplet Of St Michael
- Palace Cinema Central Park Sydney
- Pin Oak Court In Vermont South
- 35 Gawler Street Mount Barker
