SOLVED 2. Calculate the number of moles of NaHCO that were required3 to neutralize the HC2H3O2
Figure \(\PageIndex{3}\): Distilled white vinegar is a solution of acetic acid in water. Solution. As in previous examples, the definition of molarity is the primary equation used to calculate the quantity sought. In this case, the mass of solute is provided instead of its molar amount, so we must use the solute's molar mass to obtain the.

O Molar Mass
Molar Mass, Molecular Weight and Elemental Composition Calculator. Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of ethanoic acid (CH 3 COOH) is 60.0520 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between CH3COOH weight and moles. Compound. Moles.
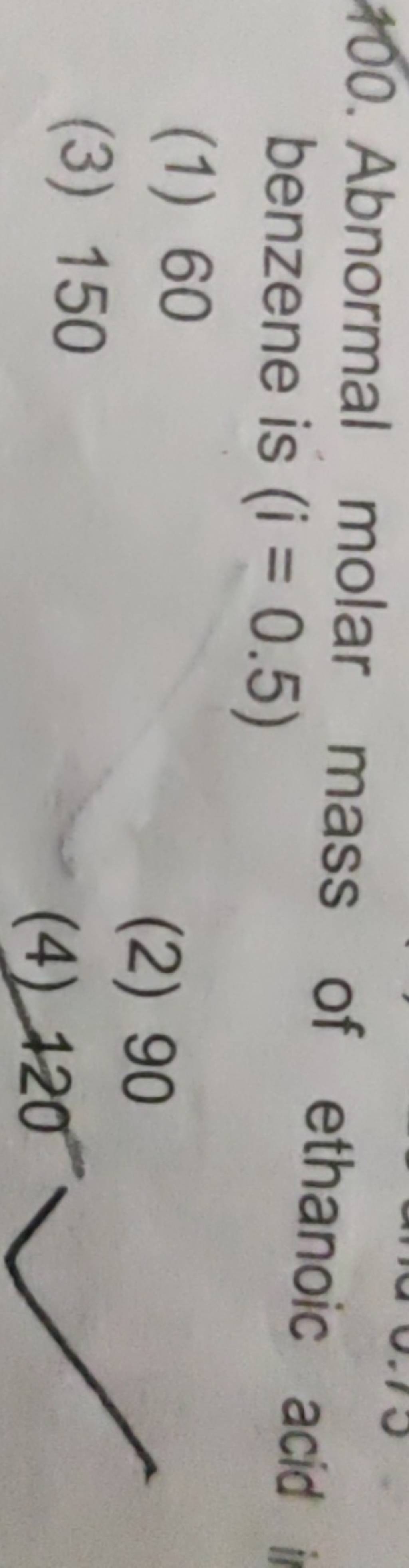
Abnormal molar mass of ethanoic acid benzene is (i=0.5) Filo
Acetic Acid | CH3COOH or C2H4O2 | CID 176 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.

[Chemistry] Differentiate between Ethanol and Ethanoic acid Class 10
Acetic acid / ə ˈ s iː t ɪ k /, systematically named ethanoic acid / ˌ ɛ θ ə ˈ n oʊ ɪ k /, is an acidic, colourless liquid and organic compound with the chemical formula CH 3 COOH (also written as CH 3 CO 2 H, C 2 H 4 O 2, or HC 2 H 3 O 2). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a.
Acetic Acid Definition, Formula & Uses
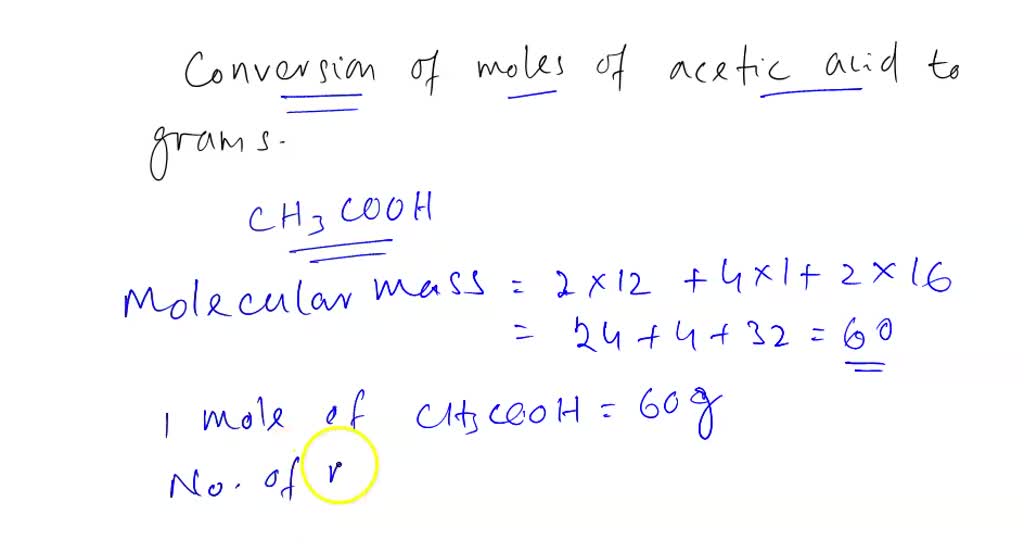
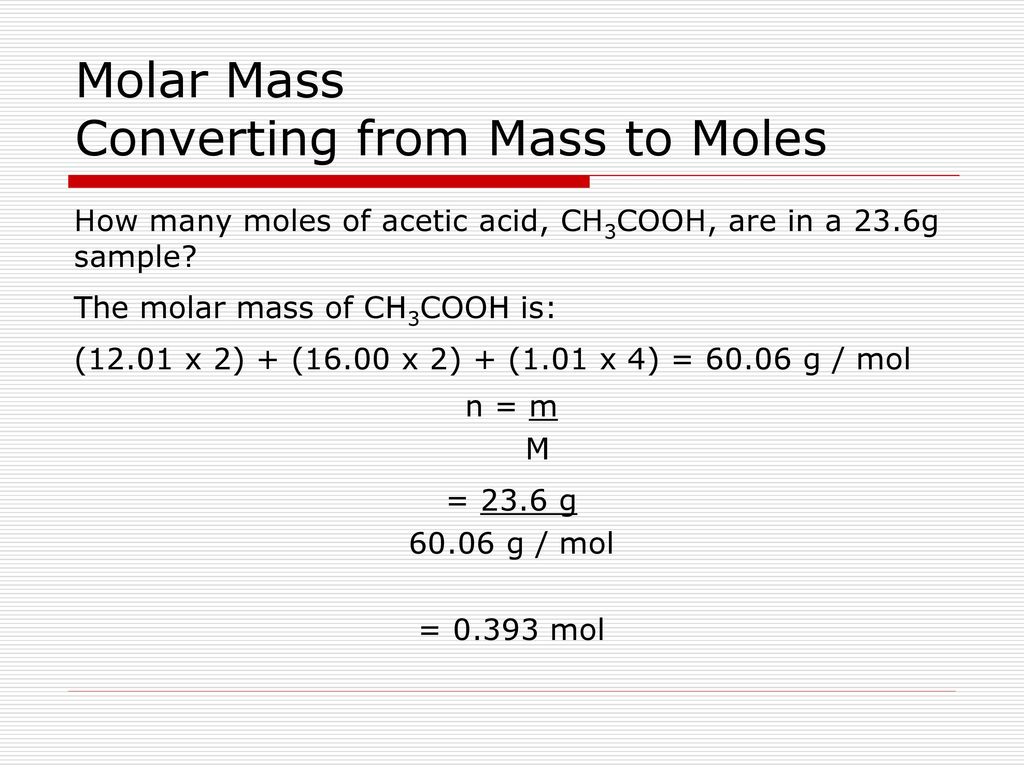
Finally, add together the total mass of each element to get the molar mass of CH3COOH: 24.0214 g/mol + 4.03176 g/mol + 31.9988 g/mol = 60.05196 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in CH3COOH, divide each total from step 3 by the total molar mass found in step 4:

Calculate the molecular mass of ethanoic acid, CH(3)COOH. (Atomic ma
Density of 2.03 molar aqueous solution of acetic acid is 1.017 g / ml . Molecular mass of acetic acid is 60. Calculate the molality of solution? Q. Determination of the molar mass of acetic acid in benzene using freezing point depression is affected by: View More.

Calculate the molecular mass of ethanoic acid, CH3 COOH. (Atomic masses
Explanation of how to find the molar mass of CH3COOH: Acetic acid.A few things to consider when finding the molar mass for CH3COOH:- make sure you have the c.

Draw the electron dot structure......" ethanoic acid " Brainly.in
Step2. The formula for molecular mass of Ethanoic acid. The formula for Ethanoic acid is CH 3 COOH; The molecular mass of CH 3 COOH = 2×The atomic mass of C + 4× The atomic mass of H + 2×Atomic mass of O; Step3. Calculation of molecular mass of Ethanoic acid. The molecular mass of Ethanoic acid = 2×12.01+ 4×1.0079+2×16 u = 60. 0516 u. ≃.

Ethanoic acid, molecular model Stock Image C029/0959 Science Photo Library
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. Calculate the molar mass of ethanoic acid in grams per mole or search for a chemical formula or substance.

Molar mass of the elements of the example Download Table
It is commonly called acetic acid. After adding 5-8% of acetic acid in water it becomes vinegar. Understand its properties, structure, chemical reactions like Esterification, Uses with FAQs of Ethanoic acid. Visit BYJU'S for more information. Login.. Molecular Weight/ Molar Mass: 60.05 g/mol: Density: 1.05 g/cm 3: Boiling Point: 118 o C.
Understanding the Significance of Acetic Acid Molar Mass
Step 2: The molar mass of carbon is approximately 12.01 g/mol, the molar mass of hydrogen is 1.008 g/mol, and the molar mass of oxygen is 16 g/mol. How do we know the molar masses of individual.
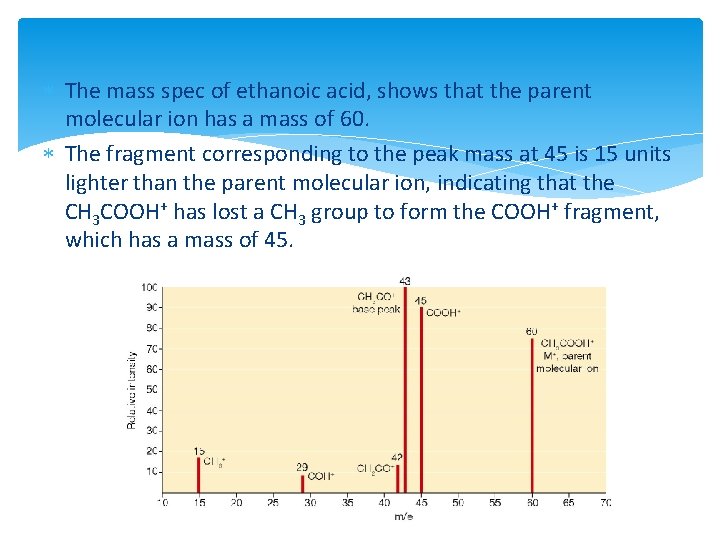
Chapter 8 Mass Spectrometry Mass Spectrometry The mass
As mass/volume = molarity × molar mass, then mass / (volume × molar mass) = molarity. Substitute the known values to calculate the molarity: molarity = 5 / (1.2 × 36.46) = 0.114 mol/l = 0.114 M. You can also use this molarity calculator to find the mass concentration or molar mass. Simply type in the remaining values and watch it do all the.
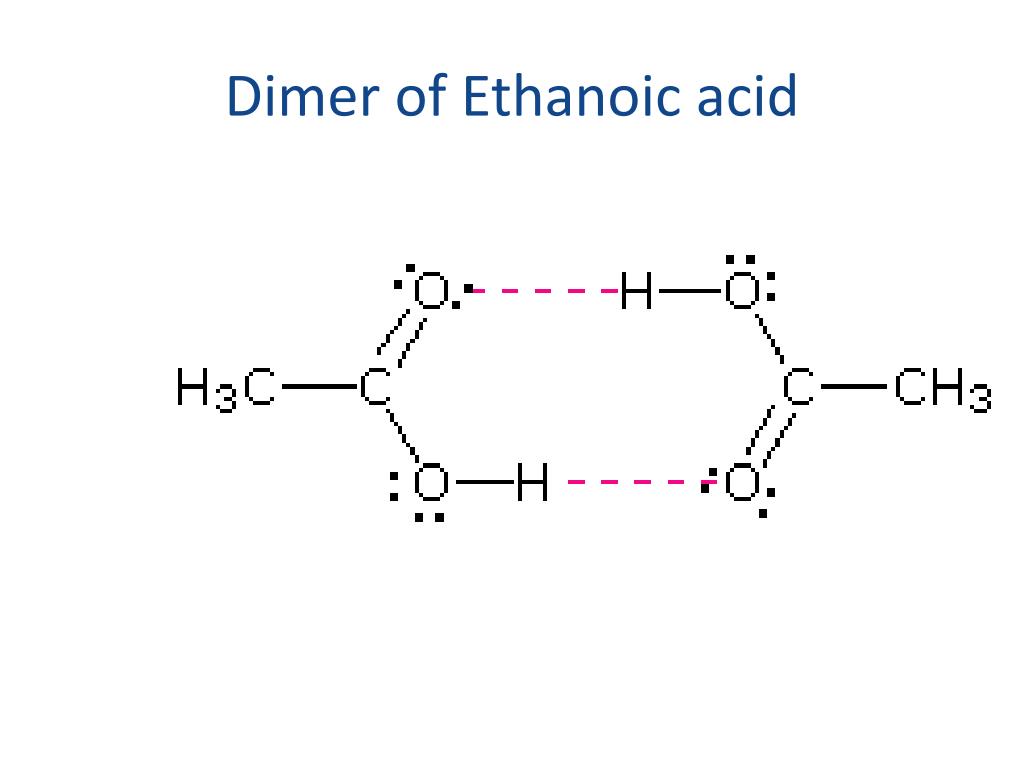
PPT Bonding Intermolecular Forces PowerPoint Presentation, free download ID1992428
Quantity Value Units Method Reference Comment; T boil: 391.2 ± 0.6: K: AVG: N/A: Average of 80 out of 90 values; Individual data points Quantity Value Units Method Reference Comment; T fus: 289.6 ± 0.5
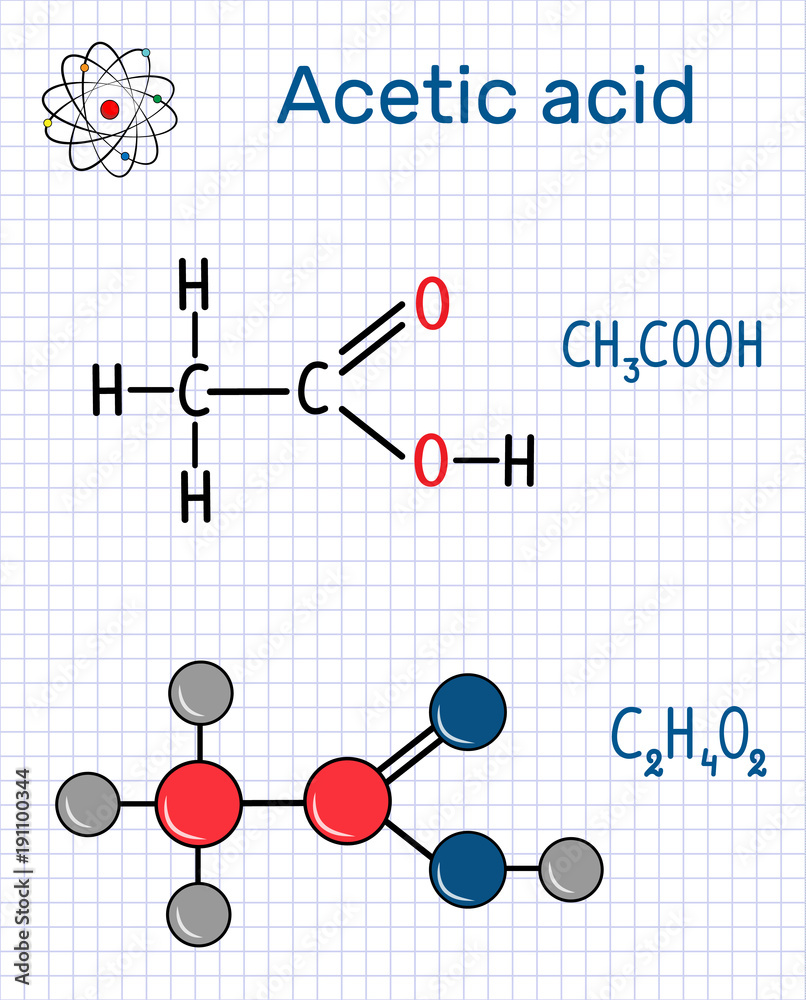
Acetic acid (ethanoic) molecule. Structural chemical formula and molecule model. Sheet of paper
"The molar mass of acetic acid is "60.05*g*mol^-1 And how did we get this quantity? All I did was take the molar mass of each element in the formula of acetic acid, weighted according to its frequency, i.e. "Molar mass" = {2xx12.011(C)+ 4xx1.00794(H)+2xx15.999(O)}*g*mol^-1 = 60.05*g*mol^-1. And can you tell me how many acetic acid molecules.

Calculate the molar mass of CH3COOH , Ethanoic acid Brainly.in
Acetic acid. Formula: C 2 H 4 O 2. Molecular weight: 60.0520. IUPAC Standard InChI: InChI=1S/C2H4O2/c1-2 (3)4/h1H3, (H,3,4) Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: QTBSBXVTEAMEQO-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet.

Acetate molar mass fasflash
The carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether. Figure 15.3.1 15.3. 1: Hydrogen Bonding between an Acetic Acid Molecule and Water Molecules. Carboxylic acids of low molar mass are quite soluble in water. Table 15.4.1 lists some physical properties for selected carboxylic acids.
