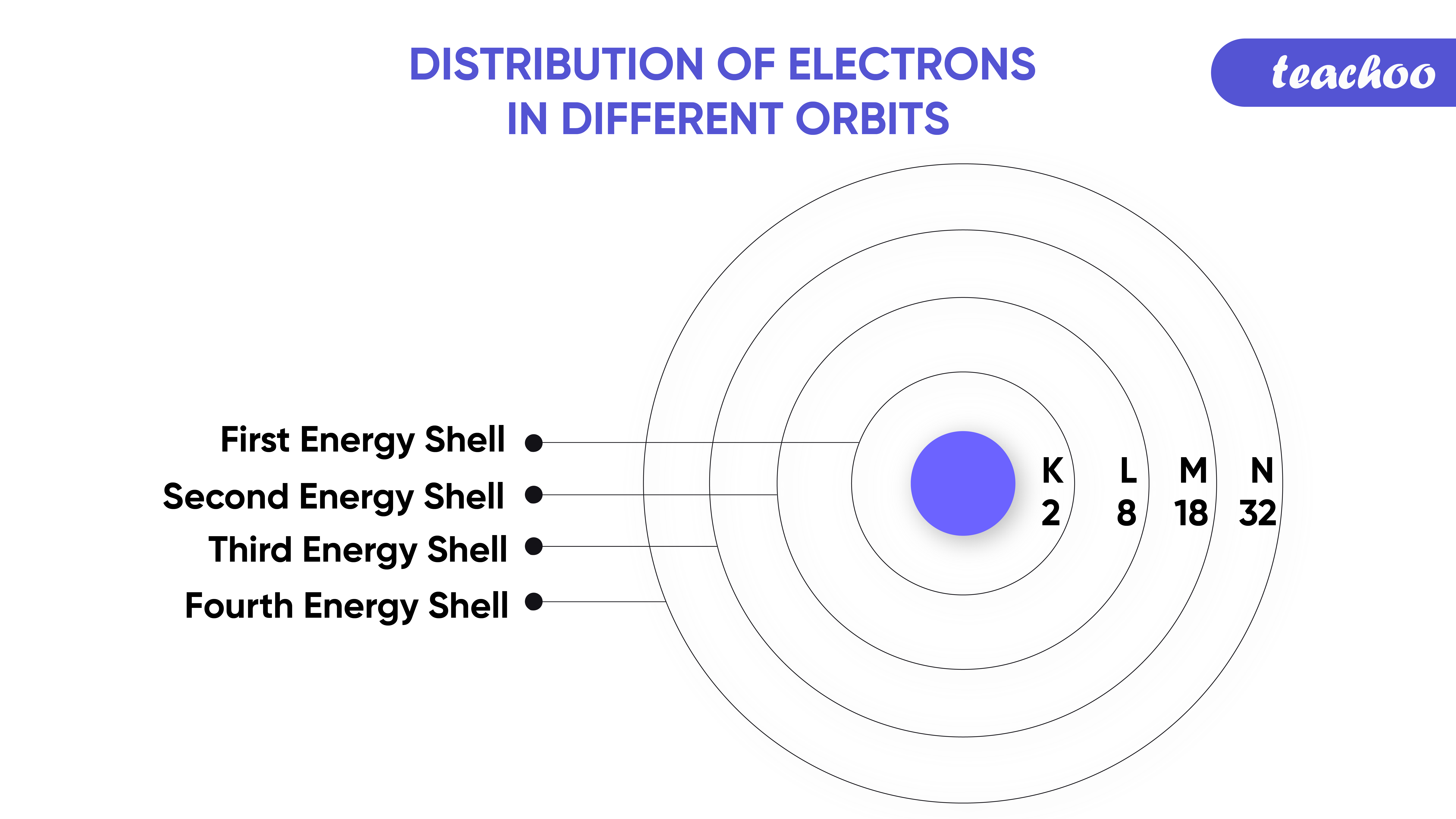
Distribution of Electrons in Different Orbits [with Examples] Teacho
The electron configuration for the first six orbitals / orbital sets that exist for an atom is shown below. 1s22s22p63s23p64s2. Note that the location of up to 20 electrons (2+2+6+2+6+2) can be specified using these six orbitals / orbital sets . As the periodic table currently contains 118 elements, this electron configuration pattern can be.
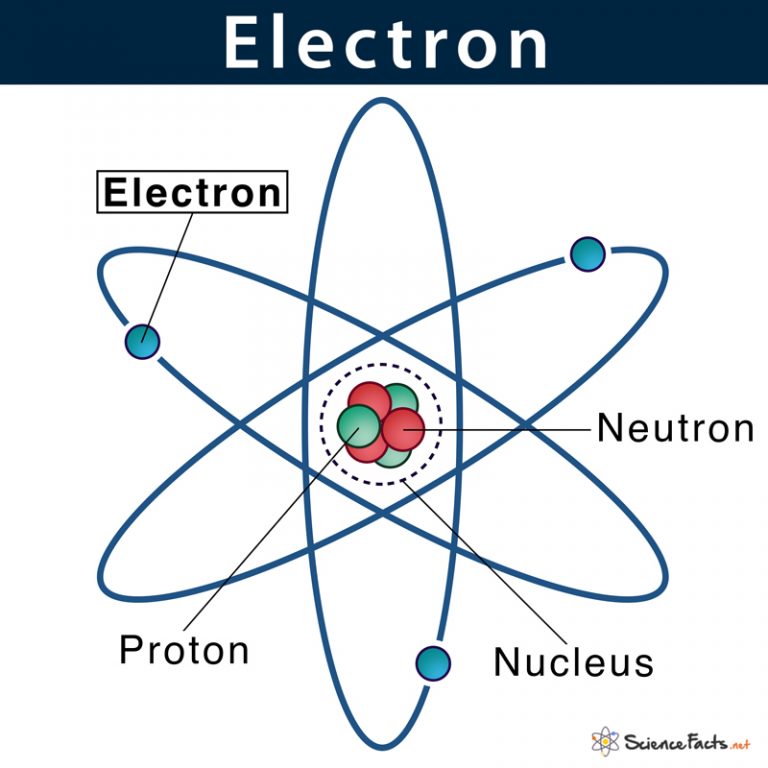
Atomic Nucleus Definition, Structure & Parts with Diagram
An electron in an atom or ion has four quantum numbers to describe its state. Think of them as important variables in an equation which describes the three-dimensional position of electrons in a given atom.. Describe the location of an electron in an atom. spin quantum number [latex](m_s)[/latex]: Describes the spin for a given electron.

How to Teach About Electrons in Atoms The Productive Teacher
Protons. Protons are the only positively charged subatomic particles in an atom. Its electrical charge is 1.6022 * 10^-19 coulomb -- the same as an electron's, although an electron's charge is negative. The proton's mass, 1.67 * 10^-27 kilograms, is very close that of a neutron, and is around 1,837 times heavier than an electron.
Atoms & Molecules echapter — The Biology Primer
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, m l = 0, m s = + 1 2 m s = + 1 2).
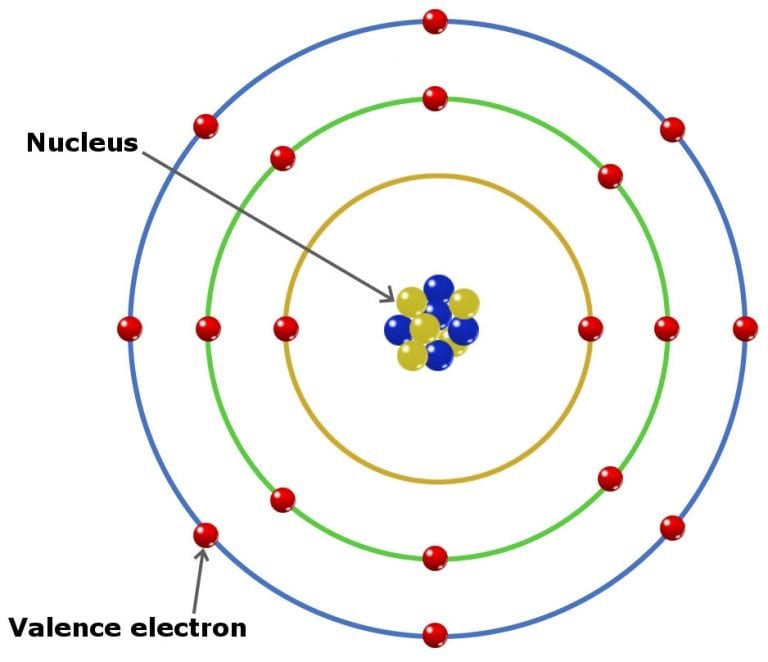
What Are Valence Electrons and How To Find Them? Where Are They Located?
In order to track down where a given electron lives in an atom, you need to know not only how far from the nucleus it is found (which determines its energy level, since electrons further out from the nucleus tend to have higher energy) but also the type of orbital that it can be found in. Think of this as knowing not only which apartment building (energy level) the electron lives in, but also.

Electrons — Structure & Properties Expii
v. t. e. The electron (. e−. or. β−. ) is a subatomic particle with a negative one elementary electric charge. [13] Electrons belong to the first generation of the lepton particle family, [14] and are generally thought to be elementary particles because they have no known components or substructure. [1]
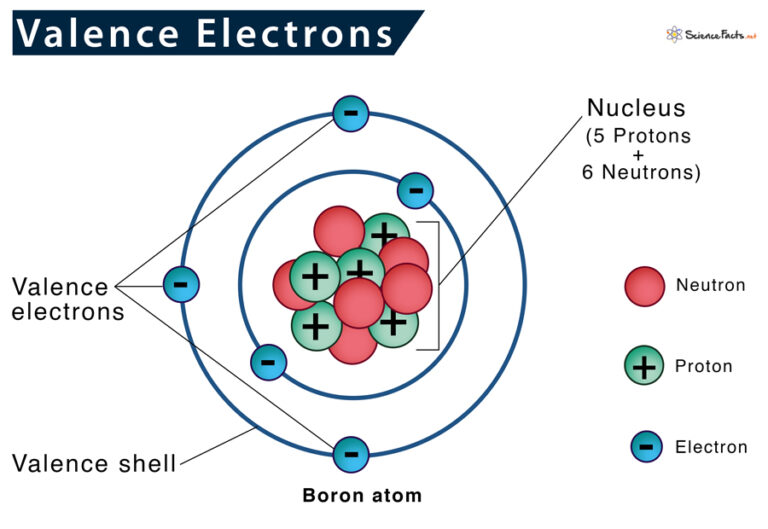
Valence Electrons Definition, Location, Importance, and Diagram
You will discover that knowing how to use the periodic table is the single most important skill you can acquire to understand the incredible chemical diversity of the elements. 8.1: Electromagnetic Radiation. 8.2: Atomic Spectra. The photoelectric effect provided indisputable evidence for the existence of the photon and thus the particle-like.
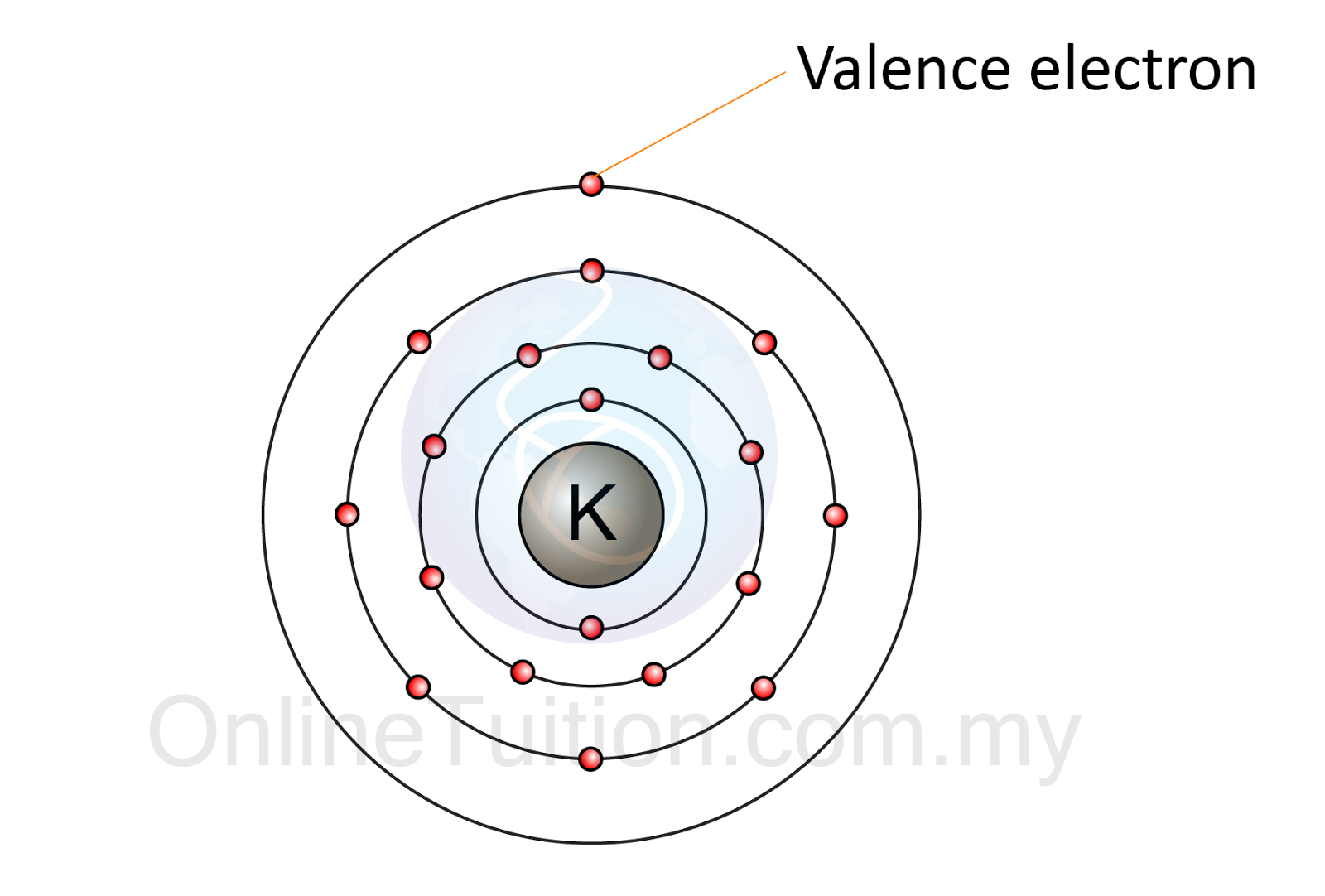
Electron Arrangement in Atom SPM Chemistry
The atom is such an important component of nature that many prominent scientists have theorized how it is made up. The discovery of subatomic particles -- protons, neutrons and electrons -- did not settle the matter. In the early 1900s, the "Plum Pudding Model" depicted protons and electrons evenly distributed throughout the atom.

Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind the Atoms Together
The goal of this section is to understand the electron orbitals (location of electrons in atoms), their different energies, and other properties. The use of quantum theory provides the best understanding to these topics. This knowledge is a precursor to chemical bonding. As was described previously, electrons in atoms can exist only on discrete.

Learn the Parts of an Atom
They are effectively a map of the electrons for a given atom. We look at the four quantum numbers for a given electron and then assign that electron to a specific orbital in the next Module. 5.14: Quantum Numbers We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom.

List of Electron Configurations of Elements
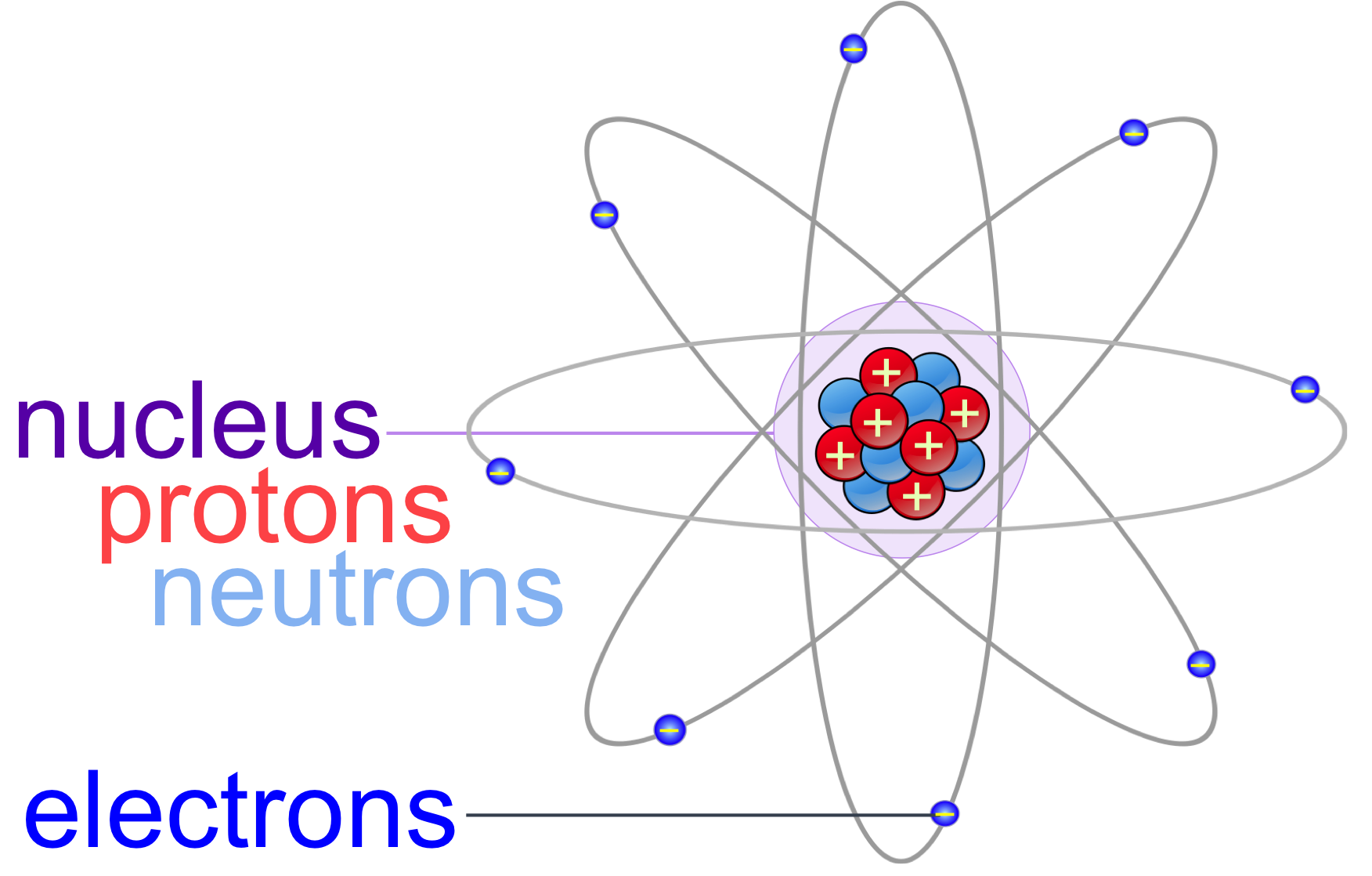
Figure 3.3.2 3.3. 2: Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Masses for the three subatomic particles can be expressed in amu ( atomic mass units) or grams. For simplicity, we will use the amu unit for.
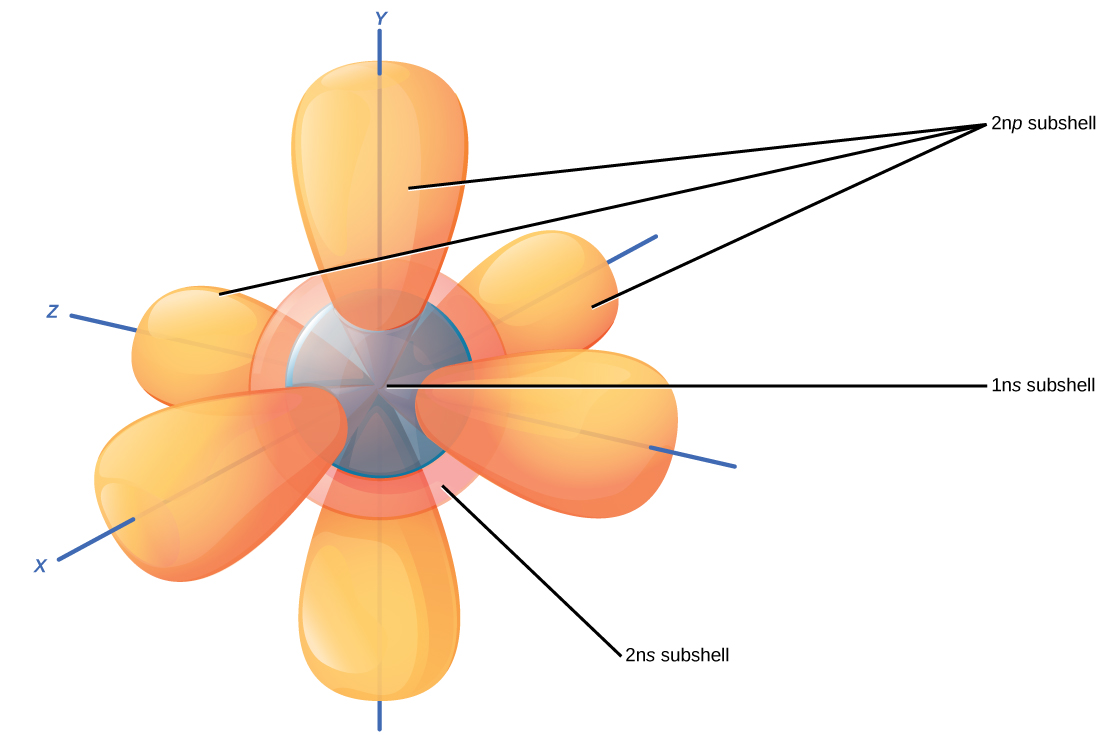
Electrons Biology for Majors I
electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic building blocks of all matter and chemistry.The negatively charged electrons circle an atom's central nucleus, which is formed by positively charged protons and the electrically neutral particles called neutrons. (The nucleus of the ordinary hydrogen atom is an exception.

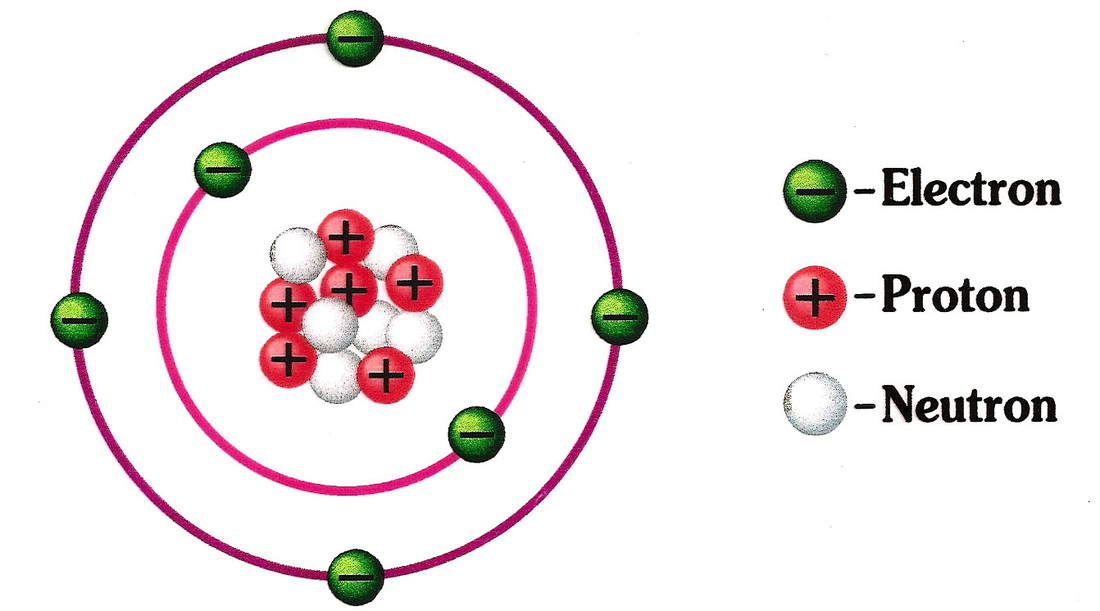
With the help of a neat labelled diagram show the location of neutron , proton and electron in
Introduction to electron configurations. Google Classroom. About. Transcript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.
Atoms and Elements Científicos Matemáticos
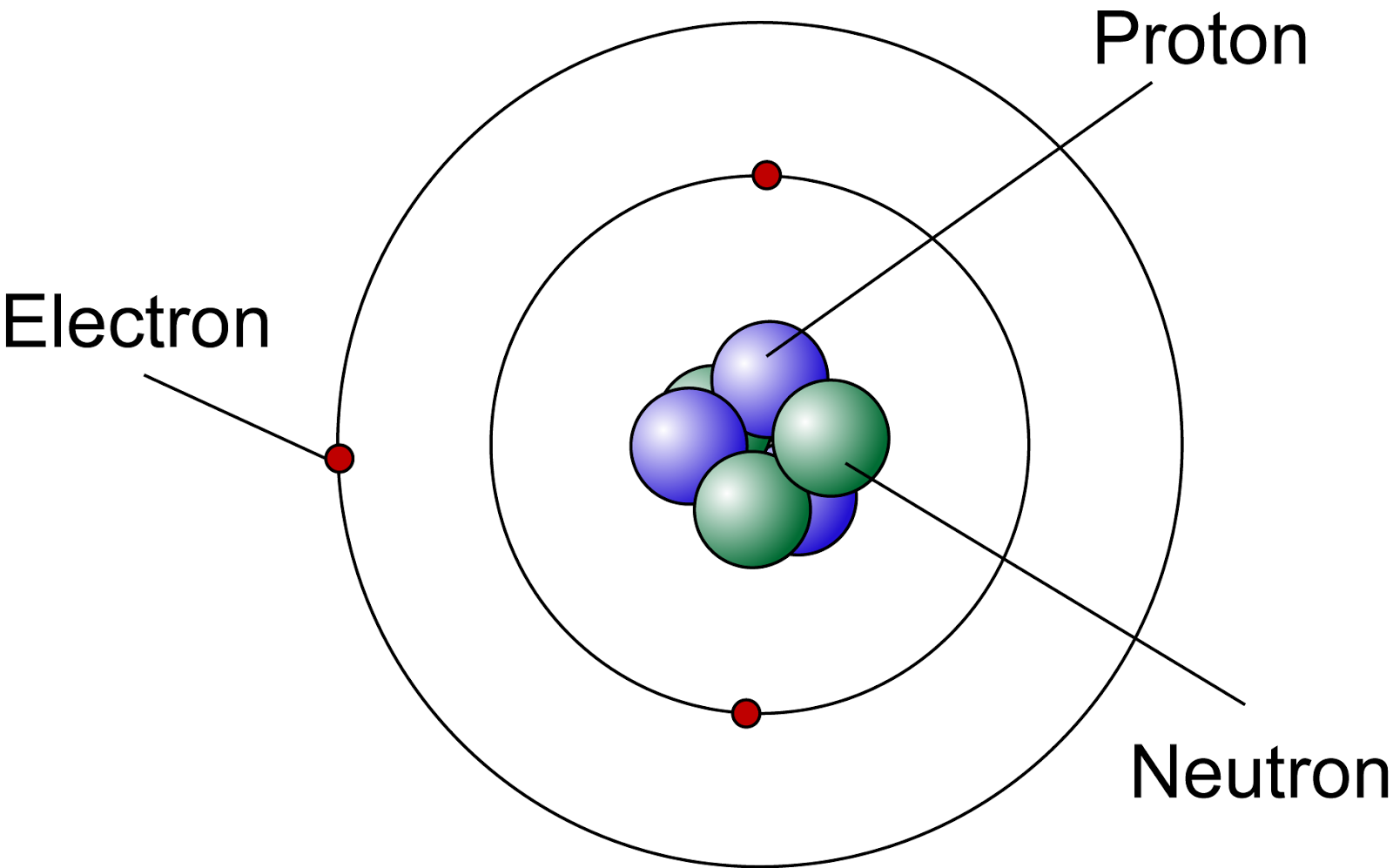
An early model of the atom was developed in 1913 by the Danish scientist Niels Bohr (1885-1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.
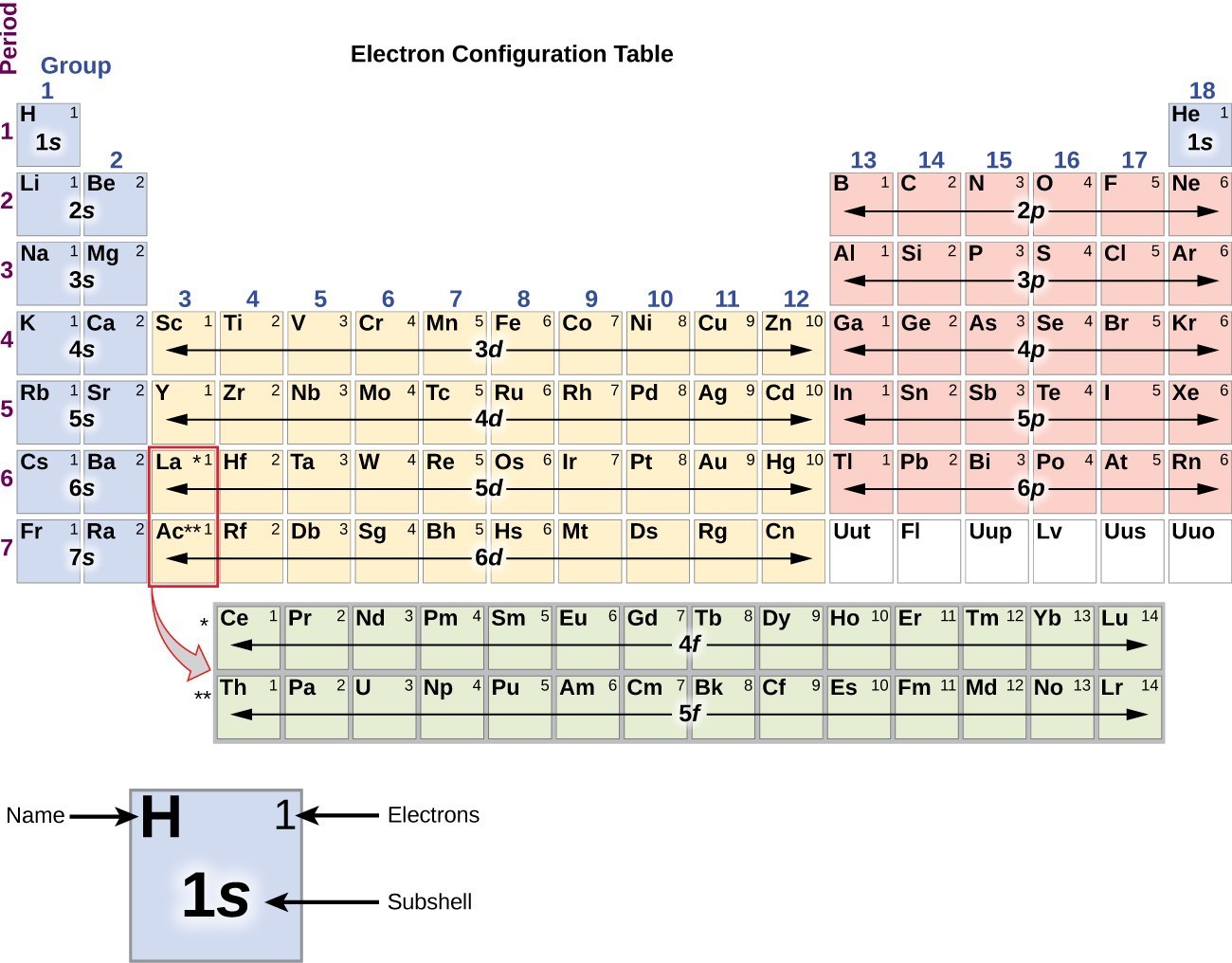
Electronic Structure of Atoms (Electron Configurations) Chemistry
Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Electrons are found outside the nucleus. Protons are positively charged and have a mass of about 1 u. Neutrons are neutral (have no charge) and also have a mass of about 1 u. Electrons are negatively charged and have a much smaller mass of about 0.0005 u.
Lets Get Inside An Atom!! The Science Station
Figure 10.4a: Principal energy level schematic: different shells are numbered by principal quantum numbers (credit: Chemistry (OpenStax), CC BY 4.0 ). This quantum mechanical model for where electrons reside in an atom can be used to look at electronic transitions, the events when an electron moves from one energy level to another.
