
5 Steps】How Many Valence Electrons Does Carbon Have?Number of Valence Electrons in Carbon
This electron configuration is written as 1 s2 2 s1. The next element is beryllium, with Z = 4 and four electrons. We fill both the 1 s and 2 s orbitals to achieve a 1 s2 2 s2 electron configuration: When we reach boron, with Z = 5 and five electrons, we must place the fifth electron in one of the 2 p orbitals.
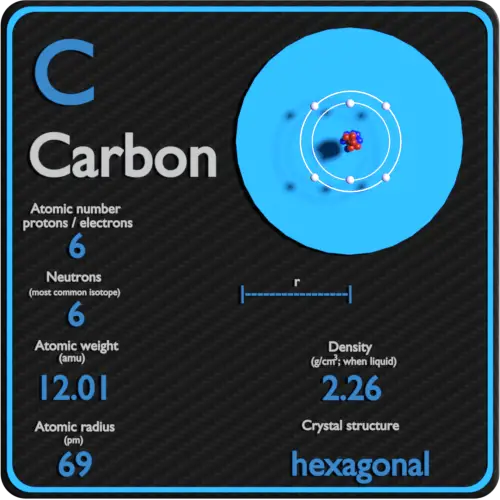
Carbon Periodic Table and Atomic Properties
The electronic configuration of carbon is 1s2 2s2 2p2. This means that a carbon atom has 6 electrons distributed among its various atomic orbitals. In general, the first energy level (principal quantum number n=1) can hold up to 2 electrons, which occupy the 1s subshell. The second energy level (n=2) can hold up to 8 electrons, which are.
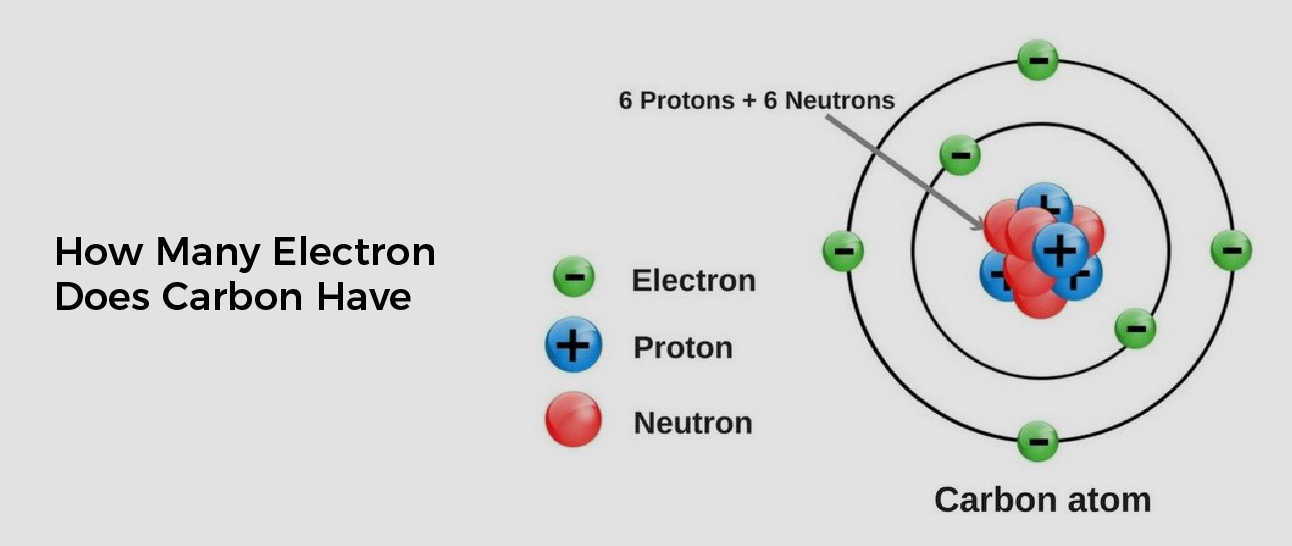
How Many Electron Does Carbon Have
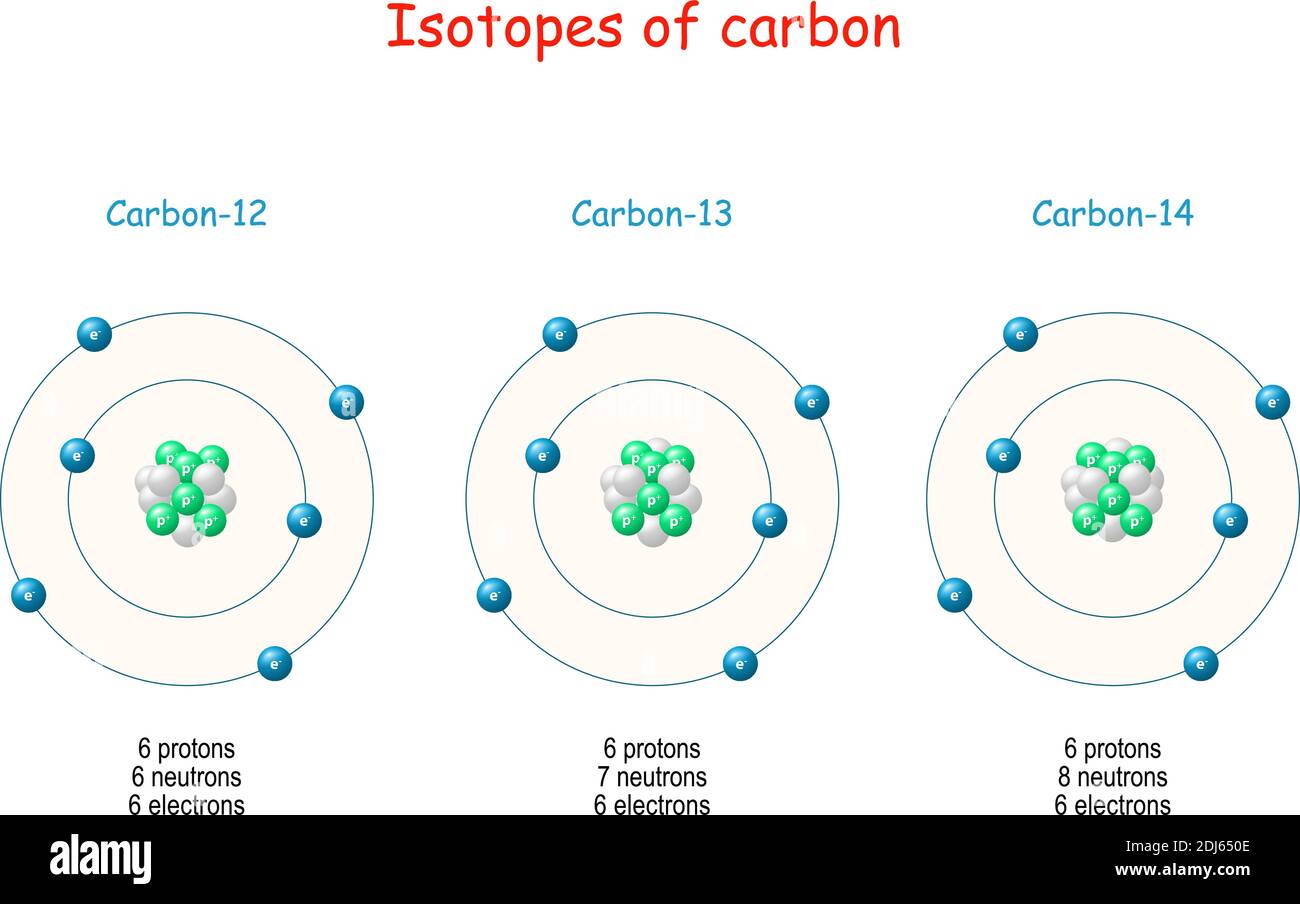
Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured, thus, its atomic mass is exactly 12 daltons by definition. Carbon-12 is composed of 6 protons, 6 neutrons, and 6 electrons. Carbon-13 is a natural, stable isotope of carbon with a nucleus containing six protons and seven.

[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds
For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.. The element carbon (\(C\)) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. In a typical sample of carbon-containing material, 98..

Carbon — Role and Importance to Life Expii
Carbon, a chemical element with the symbol C and atomic number 6, typically has six electrons. Carbon is unique because it has four valence electrons, which are the electrons in the outermost energy level of an atom. These valence electrons determine the chemical properties and reactivity of carbon.

Electrons — Structure & Properties Expii
Carbon (from Latin carbo 'coal') is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust.

Electron Configuration Of Carbon And Full Orbital Diagram
Some elements—such as carbon, potassium, and uranium—have naturally occurring isotopes. Carbon-12 contains six protons, six neutrons, and six electrons; therefore, it has a mass number of 12 (six protons and six neutrons). Carbon-14 contains six protons, eight neutrons, and six electrons; its atomic mass is 14 (six protons and eight neutrons).
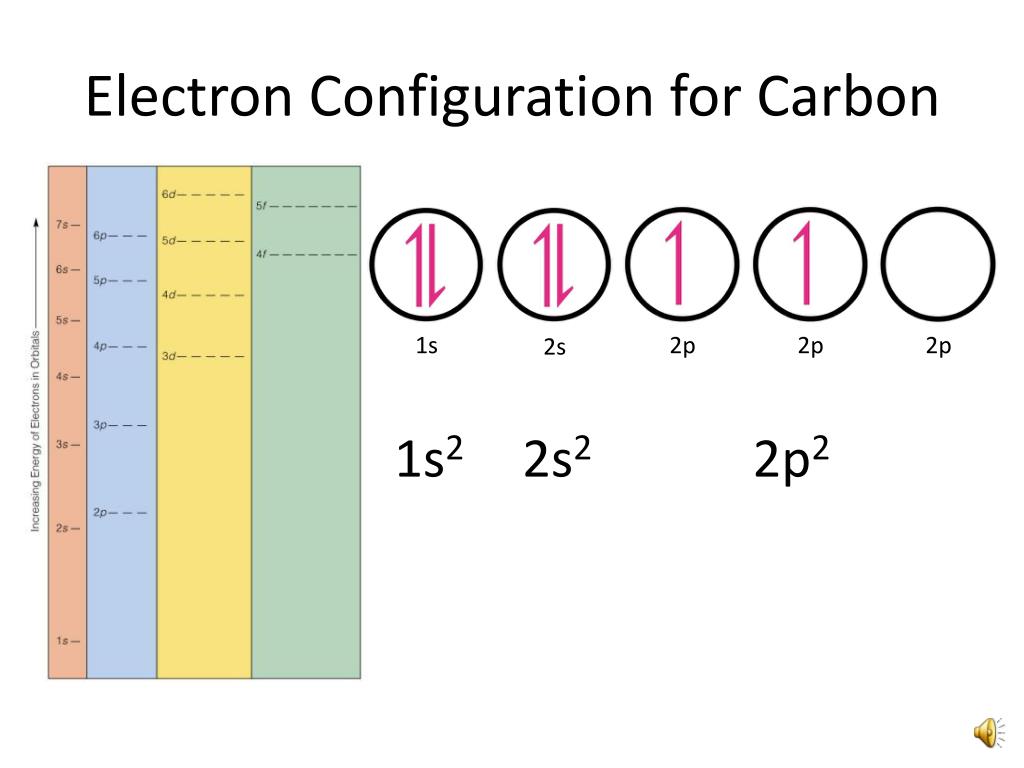
PPT Orbital Filling Electron Configurations PowerPoint Presentation ID298342
A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = 6 valence electrons Group 17 = 7 valence electrons Group 18 = 8 valence electrons (except helium)
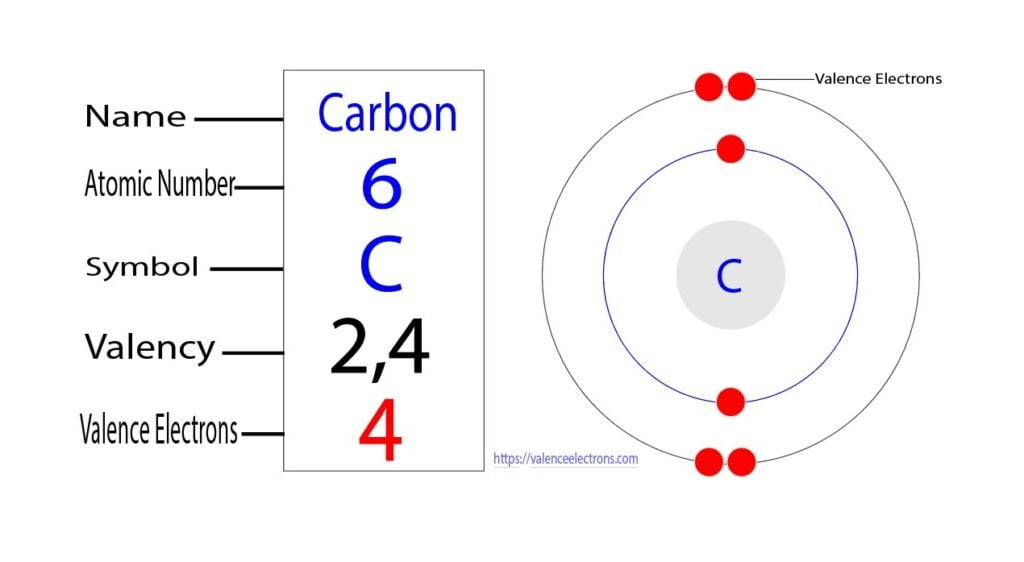
How Many Valence Electrons Does Carbon Have? Full Answer
1. Carbon forms unusually strong C-C single bonds, C=C double bonds, and carbon-carbon triple bonds. 2. The electronegativity of carbon ( EN = 2.55) is too small to allow carbon to form C 4- ions with most metals and too large for carbon to form C 4+ ions when it reacts with nonmetals. Carbon therefore forms covalent bonds with many other elements.

Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind the Atoms Together
At carbon, with Z = 6 and six electrons, we are faced with a choice.. Electrons in filled inner orbitals are closer to the nucleus and more tightly bound to it, and therefore they are rarely involved in chemical reactions. We will call these core electrons. For the representative elements (columns 1, 2, and 13-18 of the Periodic Table), the.
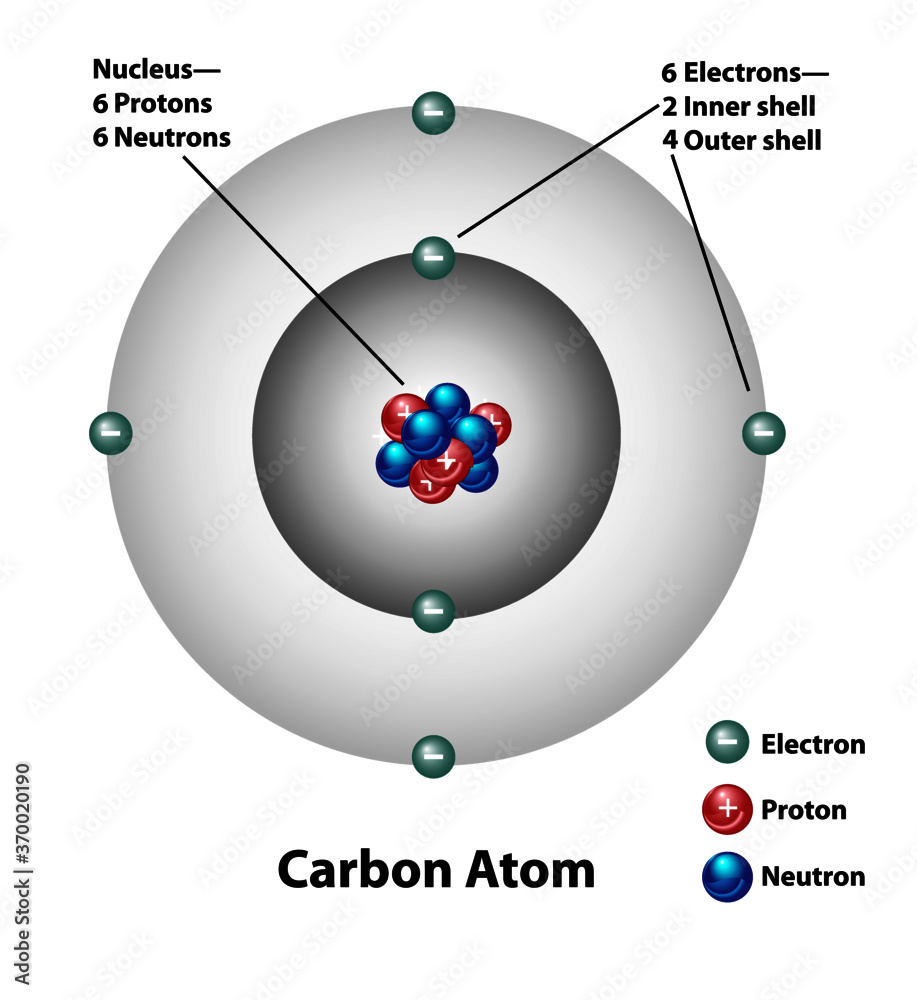
Molecular structure of a carbon atom. Electrons, protons, and neutrons are labeled. Nucleus and
The electron configuration of an atom shows the number of electrons in each sublevel in each energy level of the ground-state atom. To determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus.. carbon: 6: 1s 2 2s 2 2p.
The number of unpaired electrons in carbon atom in excited state is _____ A. 1 B. 2 C. 3 D. 4
Carbon has six electrons and the ground-state configuration 1s 2 2s 2 2p x 1 2p y 1, and so forth. Note that a superscript is used to represent the number of electrons in a particular orbital. Table 1.1 Ground-State Electron Configurations of Some Elements. Element Atomic number Configuration; Hydrogen: 1:
Carbon atomic structure hires stock photography and images Alamy
The carbon atom donates four electrons of the last shell to turn into a carbon ion (C 4+ ). In this case, the carbon atom carries a positive charge. C - 4e - → C 4+. Here, the electron configuration of carbon ion (C 4+) is 1s 2. This positive carbon ion (C 4+) has six protons, six neutrons, and two electrons.

Carbon(C) electron configuration and orbital diagram
Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) ( + 1) and a mass of 1 atomic mass unit (amu) ( amu), which is about 1.67 ×10−27 1.67 × 10 − 27 kilograms. Together with neutrons, they make up virtually all of the mass of an atom.
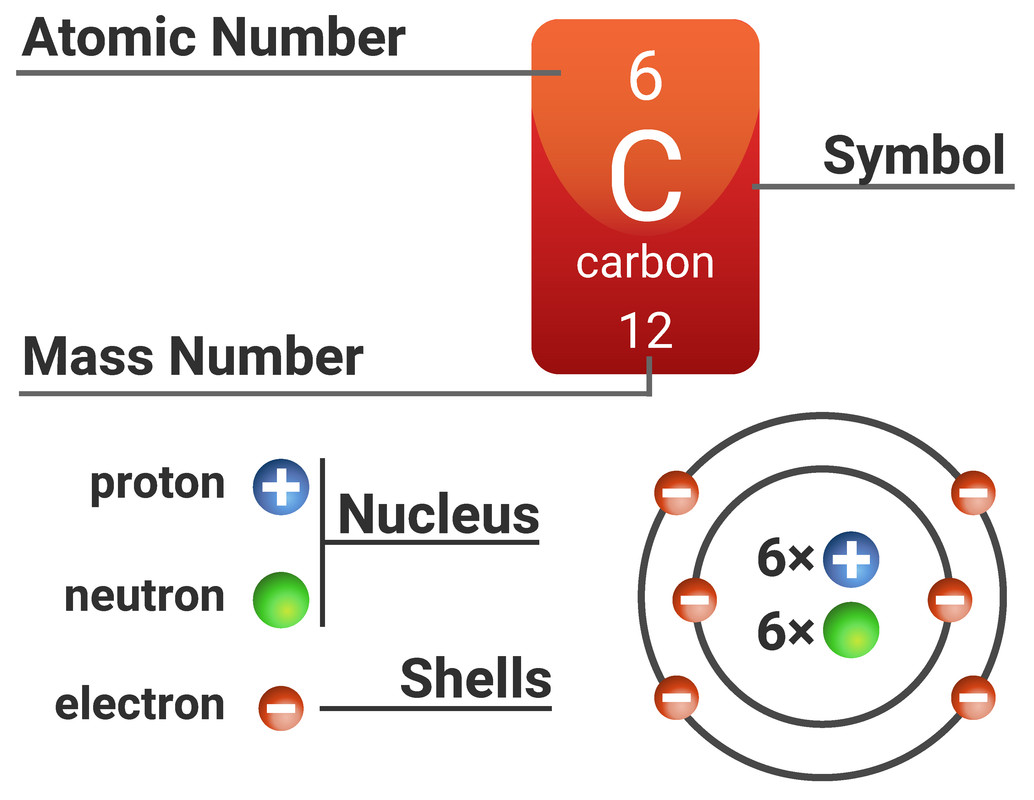
2 atomic structure UHRes wback — Postimages
Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.

Carbon Element With Reaction, Properties, Uses, & Price Periodic Table
The tiny superscripts say how many electrons live in each orbital, the letters represent the orbitals that are available, and the big numbers say which energy level the orbitals are found in. Remember that the total number of electrons just equals the total number of protons, and so the superscripts add up to 8, the atomic number of oxygen.
- What Is A Daith Piercing
- Dark Of The Moon Optimus
- Little Man J Net Worth
- Dragon Tattoo On A Woman
- Kia Stonic Car Seat Covers
- Volkswagen Polo Dashboard Warning Lights
- Whats Closed On Good Friday
- Prince Of Wales And Lady Diana 50 Cent Coin Value
- Department Of Energy Environment And Climate Action
- How To Stop Private Browsing
