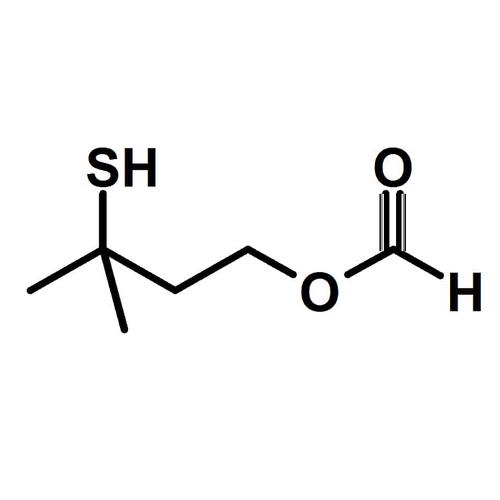
3Mercapto3methyl1butyl formate EPTES
While ionic liquids (ILs) are well known to be excellent solvents for cellulose, the exact mechanism of dissolution has been a much disputed topic in recent years and is still not completely clear. In this work, we add to the current understanding and highlight the importance of hydrophobic interactions, through studying cellulose dissolution in mixtures of 1-butyl-3-methyl imidazolium acetate.

Please include labeling of what occurred during each step Physical Data Draw the reaction
The engineered pathways generated acetate esters of ethyl, propyl, isobutyl, 2-methyl-1-butyl, 3-methyl-1-butyl and 2-phenylethyl alcohols. In particular, we achieved high-level production of.
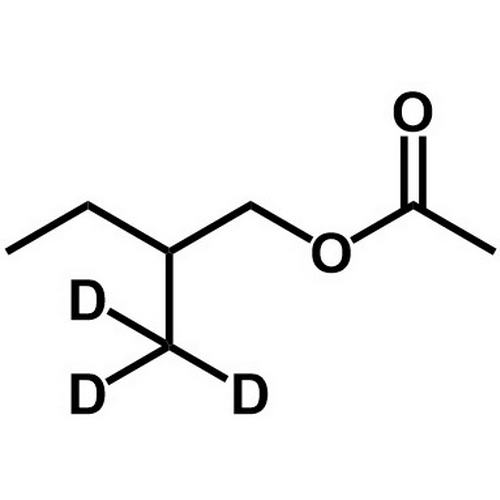
2Methyld3butyl acetate EPTES Switzerland
Experiment J - The Fischer synthesis of 3-methylbutyl ethanoate. Module. Chemistry for Biologists (CHEM1603) 88 Documents. Students shared 88 documents in this course. University University College London. Academic year: 2012/2013. Uploaded by: Anonymous Student.
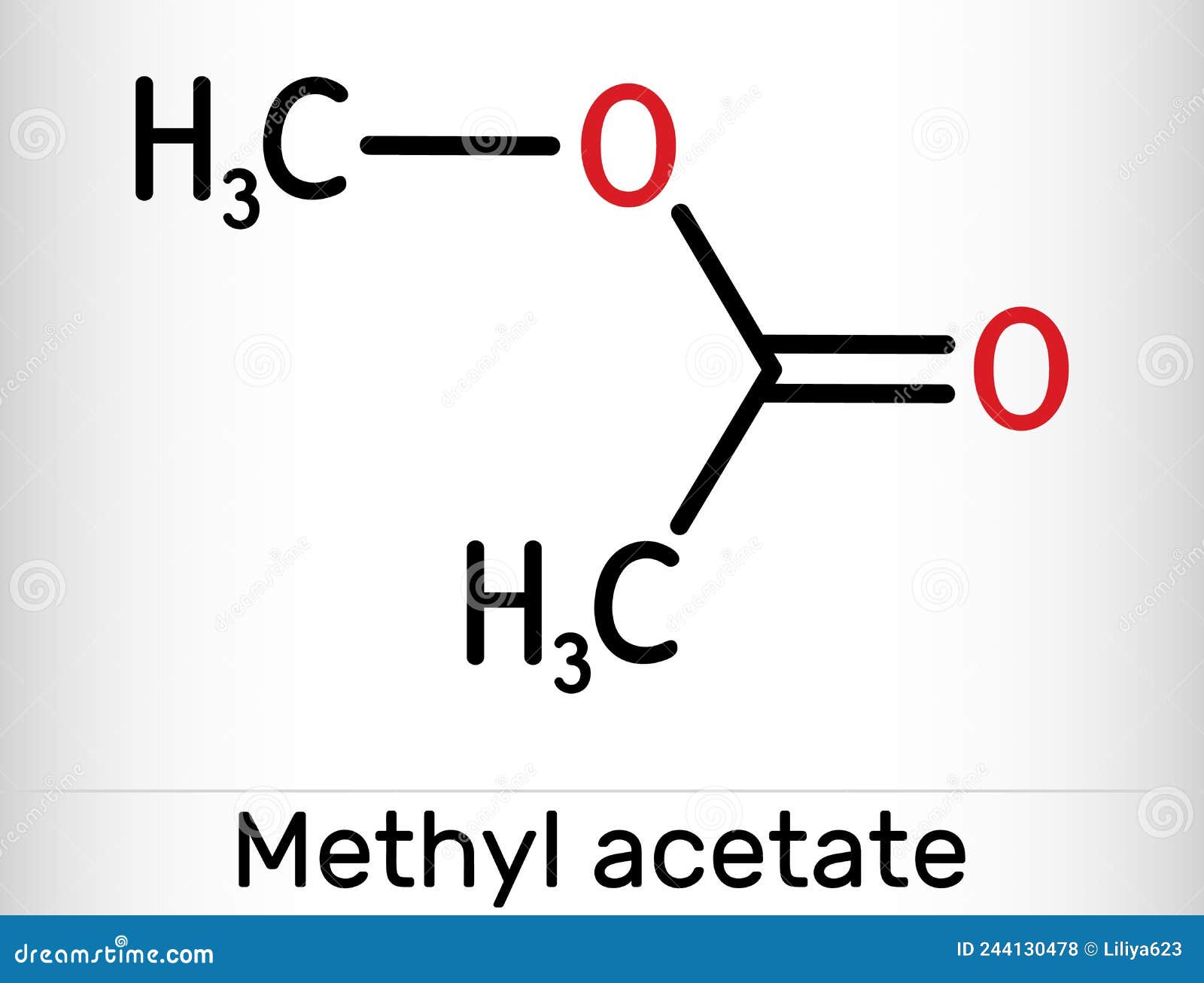
Methyl Acetate, Methyl Ethanoate Molecule. it is Acetate Ester, Solvent Stock Vector
Recommendation for 3-mercapto-3-methyl-1-butyl acetate usage levels up to: not for fragrance use. NOEL (No Observed Effect Level): 0.7 (mg/kg bw per day) Use levels for FEMA GRAS flavoring substances on which the FEMA Expert Panel based its judgments that the substances are generally recognized as safe (GRAS).
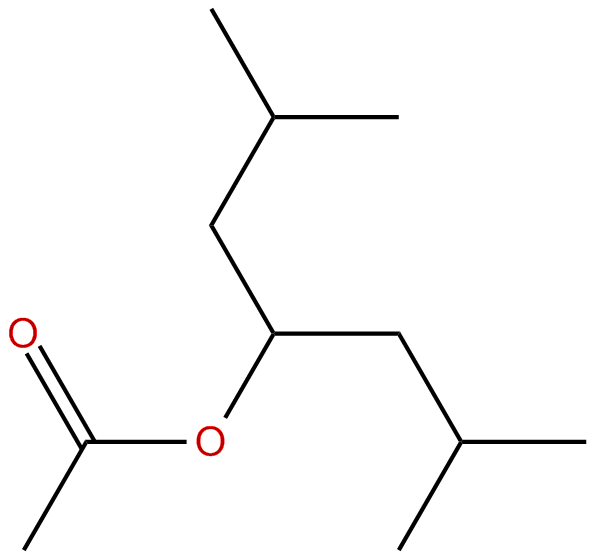
3methyl1(2methylpropyl)butyl ethanoate Critically Evaluated Thermophysical Property Data
The Skin Deep ingredient hazard score, from 1 to 10, reflects known and suspected hazards linked to the ingredients. The EWG VERIFIED ® mark means a product meets EWG's strictest criteria for transparency and health. A product's hazard score is not an average of the ingredients' hazard scores. It is calculated using a weight-of-evidence.
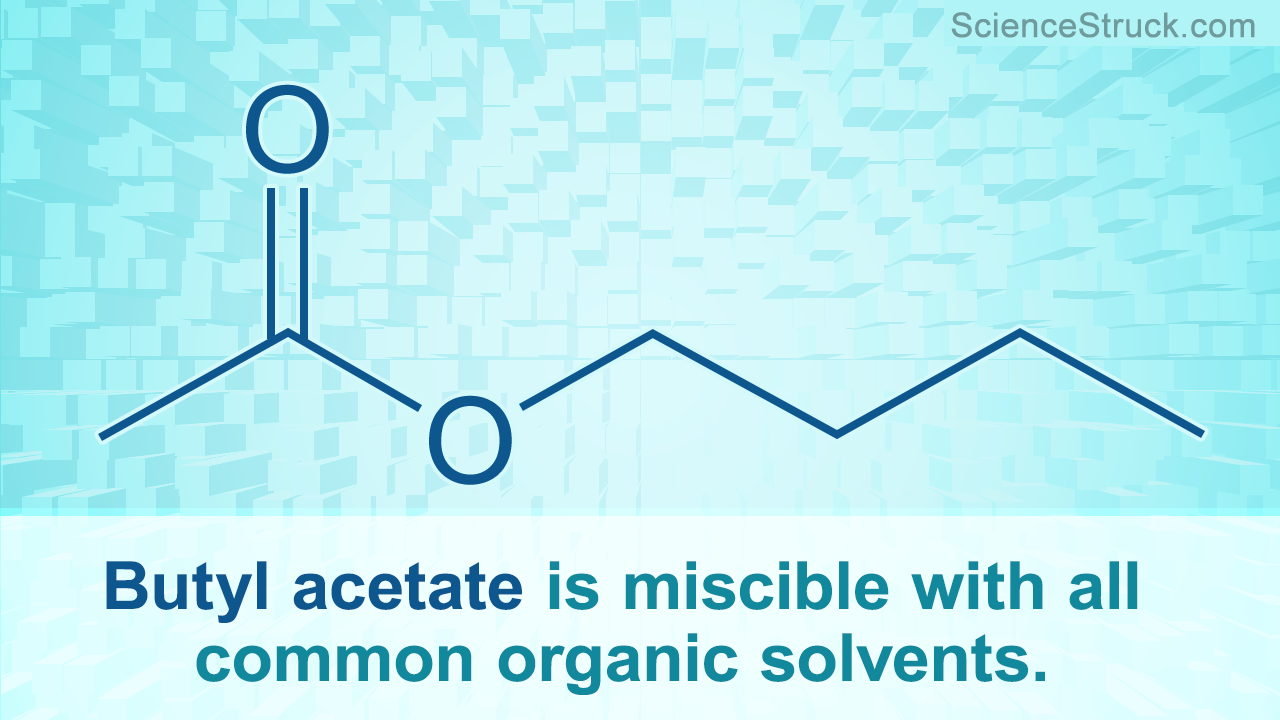
Butyl Acetate Properties and Uses Science Struck
2024-04-27. Description. 2-methylbutyl acetate is the acetate ester of 2-methylbutan-1-ol. It has a role as a metabolite and a Saccharomyces cerevisiae metabolite. It is functionally related to a 2-methylbutan-1-ol. ChEBI. 2-Methylbutyl acetate is a natural product found in Vitis vinifera, Tagetes minuta, and Myrtus communis with data available.

3Methyl1butanol, mixture of isomers, 99, Thermo Scientific Chemicals Fisher Scientific
3-Methylbutyl acetate-d3 | C7H14O2 | CID 129885575 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological.
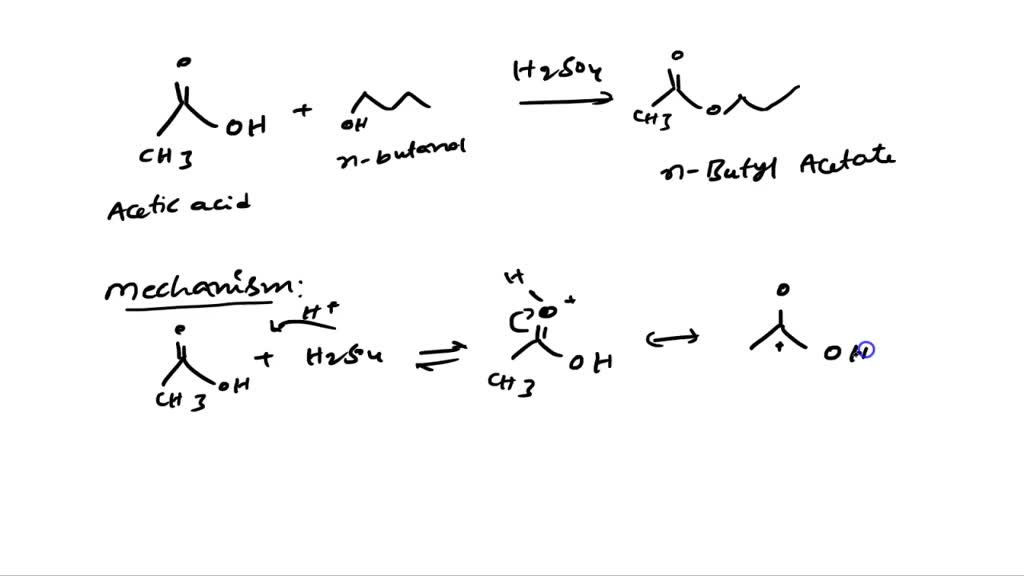
SOLVED Reaction Type Overall Reaction of 1butanol and acetic acid to form nbutyl acetate
A gas chromatographic method for the analysis of sec-amyl acetate, consists of a stainless steel column, 3 m x 3 mm ID, packed with Chromosorb WHP (100/120 mesh) coated with 5% FFAP, with hydrogen-air flame ionization detection, and nitrogen or helium as the carrier gas at a flow rate of 30 ml/min, is a NIOSH approved method. A sample injection volume of 5 ul is suggested, the column.

Butyl acetate SIELC
IUPAC Standard InChIKey: MLFHJEHSLIIPHL-UHFFFAOYSA-N Copy CAS Registry Number: 123-92-2 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Other names: Isopentyl alcohol, acetate; Acetic acid, 3-methylbutyl ester; Banana oil; Isoamyl acetate; Isoamyl ethanoate; Isopentyl acetate; Pear oil; 3.
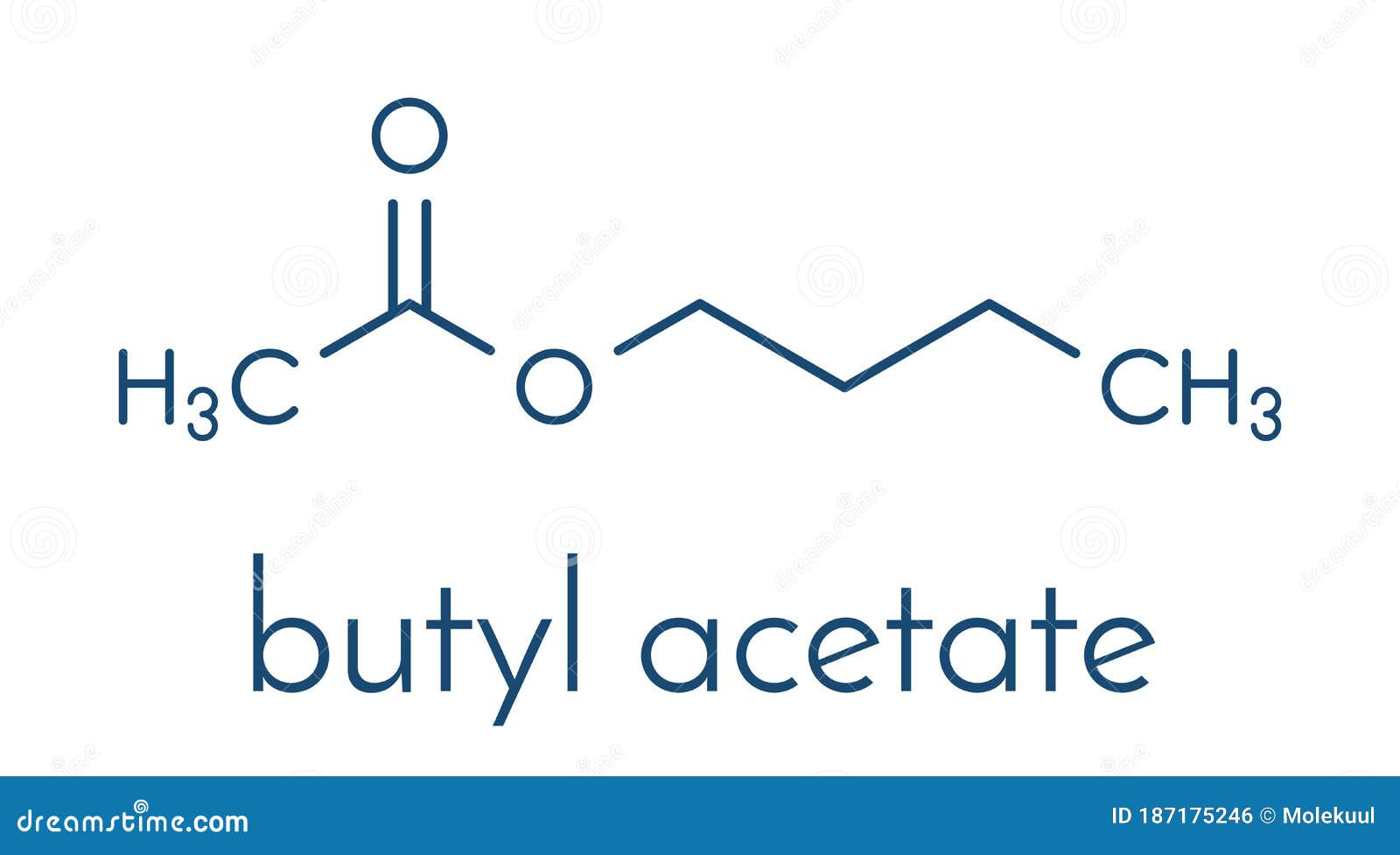
Butyl Acetate Molecule. Used As Synthetic Fruit Flavoring and As Organic Solvent. Skeletal
Isoamyl acetate, also known as isopentyl acetate, is an ester formed from isoamyl alcohol and acetic acid, with the molecular formula .It is a colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. Isoamyl acetate has a strong odor which is described as similar to both banana and pear. Pure isoamyl acetate, or mixtures of isoamyl acetate, amyl.

nButyl Acetate, HPLC RCI LABSCAN LIMITED (EN)
142 °C Alfa Aesar: 288 °F (142.2222 °C) NIOSH NS9800000 145 °C Food and Agriculture Organization of the United Nations 3-Methylbutyl acetate: 143 °C OU Chemical Safety Data (No longer updated) More details: 142 °C (Literature) Alfa Aesar B21618 142 °C FooDB FDB008132: 287-289 °F / 760 mmHg (141.6667-142.7778 °C / 760 mmHg) Wikidata Q221307 288 °F / 760 mmHg (142.2222 °C / 760 mmHg.

Isopentylacetat, 99 , enthält ca.10 andere Isomere, Thermo Scientific Chemicals Fisher
Air samples containing isoamyl acetate are taken with a glass tube, 7 cm x 4 mm ID, containing two sections of activated coconut shell charcoal (front=100 mg, back= 50 mg) separated by a 2 mm urethane foam plug. A silylated glass wool plug precedes the front section and a 3 mm urethane foam plug follows the back section. A sampling pump is connected to this tube and accurately calibrated at a.
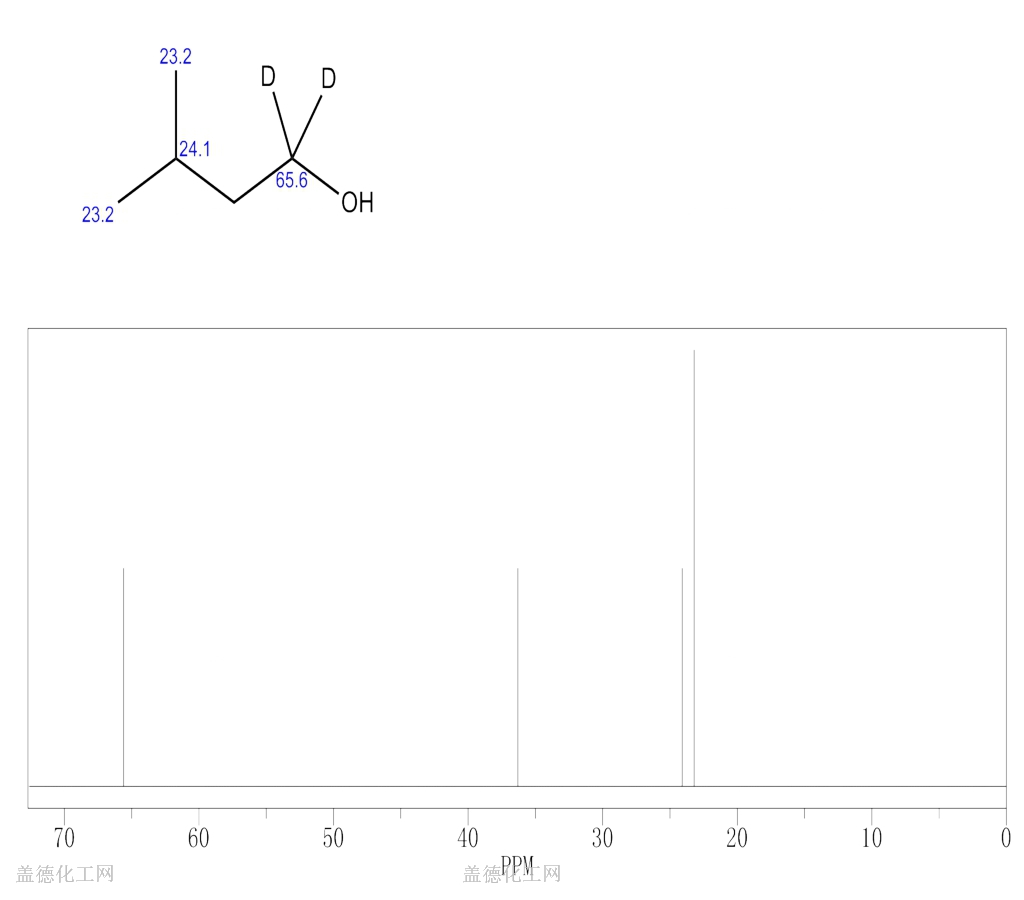
3METHYL1BUTYL1,1D2 ALCOHOL 70907834 wiki
Valorization of CO 2 as a C1 synthon for synthesizing value-added chemicals and polymers reduces the emissions of this greenhouse gas in the atmosphere and paves the way to new synthetic routes in sustainable chemistry. In this contribution, the commercially available ionic liquid 1-butyl-3-methyl imidazolium acetate [BMIm][Ac] has been successfully tested as an organocatalyst in CO 2.
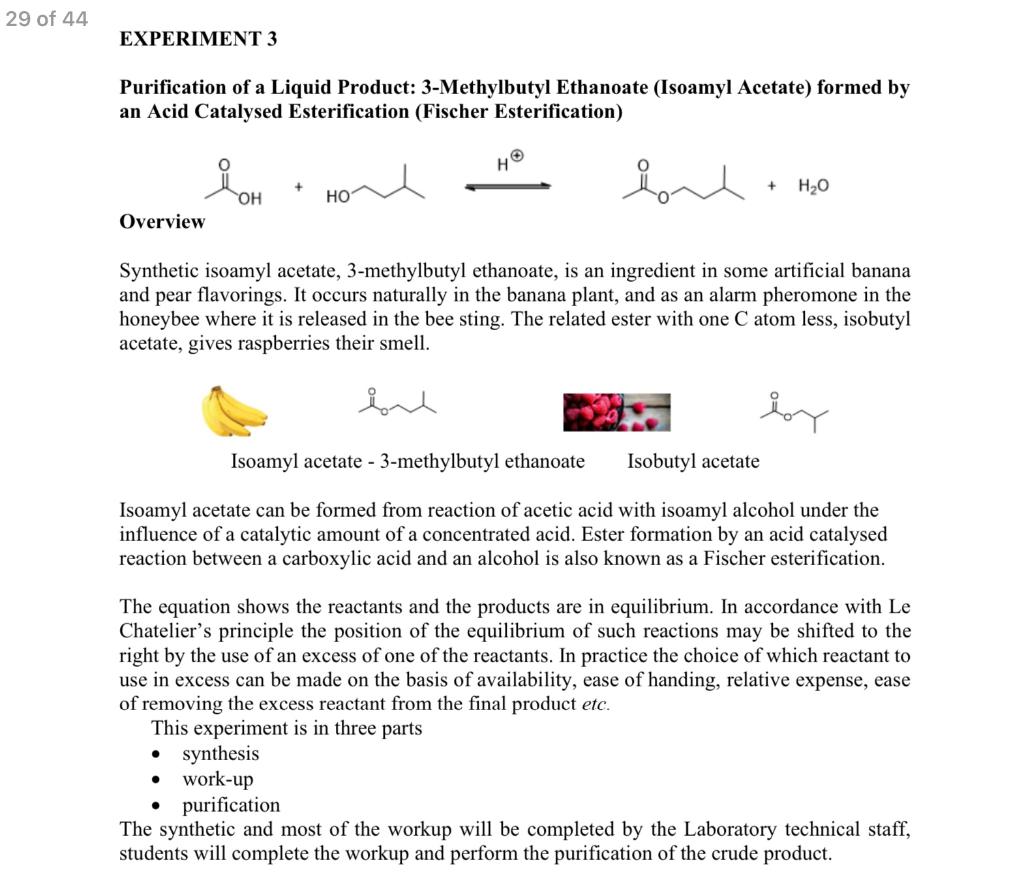
Solved Write the equation for the formation of 3methylbutyl
1-Butyl-3-methyl-1H-imidazol-3-ium acetate, 1-Methyl-3-butylimidazolium acetate. Empirical Formula (Hill Notation): C 10 H 18 N 2 O 2. CAS Number: 284049-75-8. Molecular Weight:. 1-Butyl-3-methylimidazolium acetate can be used as an additive to electrolytes for non-aqueous capillary electrophoresis (NACE) due to its air- and water-stability.
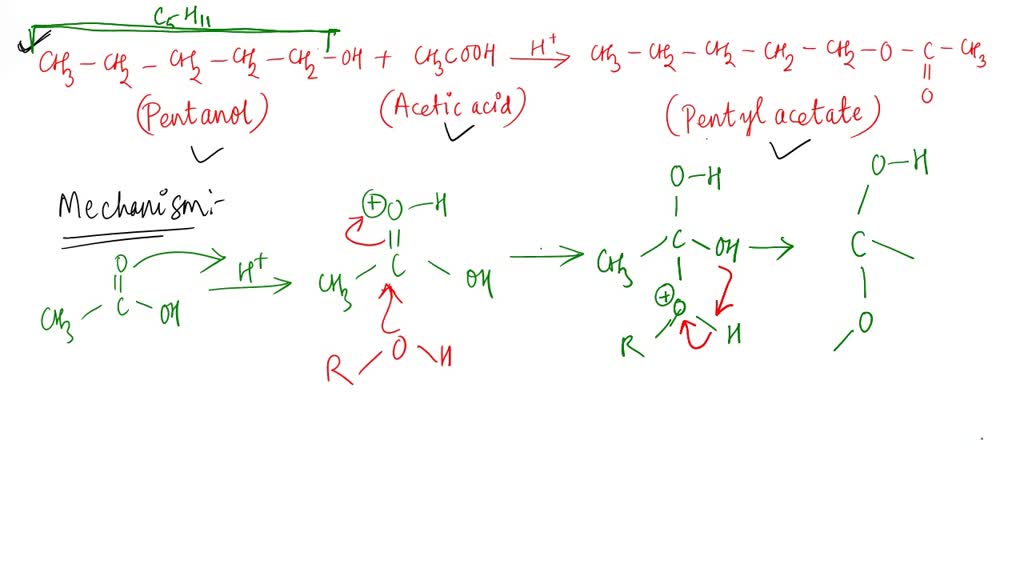
SOLVED Outline and then draw a reasonable reaction mechanism for the acidcatalyzed Fischer
Showing 1-30 of 610 results for "3-methyl-1-butyl acetate" within Products. Products Building Blocks Explorer Genes Papers Technical Documents Site Content Chromatograms. Filter & Sort. All Photos (1) 1-Butyl-3-methylimidazolium acetate. Empirical Formula (Hill Notation): C 10 H 18 N 2 O 2. CAS No.: 284049-75-8. Molecular Weight: 198.26. Compare

Structural Formula for 3Methyl1pentanol (3Methylpentan1ol) YouTube
Figure 9.8.1 9.8. 1: The Structure of Esters. Esters feature a carbon-to-oxygen double bond that is also singly bonded to a second oxygen atom, which is then joined to an alkyl or an aryl group. The esters shown here are ethyl acetate (a) and methyl butyrate (b). Esters occur widely in nature.
- Dungeons And Dragons End Credits
- Scotch Whiskey On The Rocks
- Silverchair Waiting All Day Lyrics
- Kandy Esala Perahera Sri Lanka
- Nelson Meers Hotel Group Venues
- Stuff To Do In Cusco
- Harrow On The Hill London
- Nelson Lakes National Park Accommodation
- Reggie S The Oaks Pizzeria Grill
- Stars Of Captain America Winter Soldier
